Plant organelle/membrane isolation
- Arabidopsis lumen extraction
- Arabidopsis thylakoid extraction
- BBY preparation
- Chlorophyll measurements
- Intact chloroplast isolation method
- Mitochondrial fraction
- Nuclear fraction
- Plasma membrane fraction
- PSII RC extraction for cryo-EM
- Thylakoid extraction
- Vacuol isolation
- Collection of articles

- Diatom protein extraction
- Extraction of leaf proteins
- Phenol protein extraction
- Ponceau membrane staining
- Protein extraction from grasses
- TCA acetone precipitation method
- Western blot protocol
- Western blot video tutorial
- Western blot troubleshooting

- Western blot using IgY
- Western blot in denatured conditions (urea gel)

- Peptide neutralization/competition assay
- Quantitative Western blot
- Quantitative Western blot video tutorial
- Simultaneous Western blot
- Anti-KLH antibody removal

- Dot blot
- ELISA
- Immunohistochemistry
- Immunoprecipitation
- Immunoprecipitation/IgY
- Meiotic staining
- Yolk delipidation
Technical information
Antibody typesPurification
- Antibody purification
- Antibody purification - small amount of protein
- Elution of antibodies from affinity columns
- IgY purification methods
- Protein purification using antibodies
Protocols > TCA acetone precipitation methodTCA/Acetone protein precipitation method for plant proteins.Procedure
ExampleThis method allows high protein load per well. An example of Western blot results obtained with this method can be found below.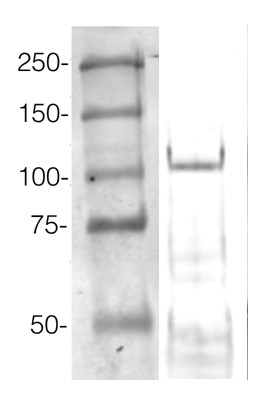 360 µg/well of Arabidopsis thaliana protein extracted by TCA-acetone precipitation from floral tissue and saturated in 8 M urea were separated on 15% SDS-PAGE and blotted for 1 h to 0.2 µm nitrocellulose at 100 V using a wet transfer system. Blots were blocked with 0.5% cold fish gelatin for 1 h at RT with agitation. Blot was incubated in the primary antibody at a dilution of 1:2500 for 1 h at RT with agitation. The blots were washed 3 x 15 min with TBS-TT at RT with agitation. Blots as incubated in the secondary antibody (DayLight®800) 1:5000 dilution for 30 min at RT with agitation, and washed once with TBSTT for 15 min, and once with TBST for 15 min before scanning with the ODyssey IRD scanner. Courtesy of Dr. Betty Chung and Pawel Baster, University of Cambridge, United Kingdom. |

