Target protein band is very weak, why there is a strong signal at the top of a gel. What can be a reason? | 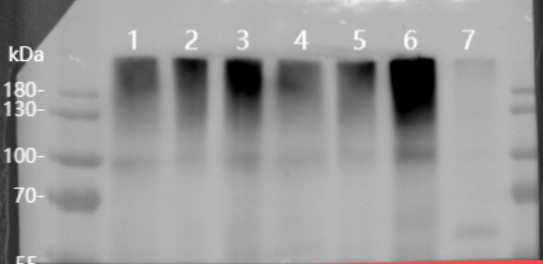 Samples: Arabidopsis thaliana total and plasma membrane protein. Target MW: 90-95 kDa (depending upon gel system used) To solve this issue: sample should be denatured at a lower temperature of 70°C. |
Latest
How to check if protein transfer from a gel to a membrane was efficient2025-04-15 How to detect membrane proteins on Western blot?
2025-03-27 Rinse Right: The Hidden Power of Washing in Western Blots
2025-02-11 Can one Western blot protocol fit all antibodies?
2025-02-07 How to reconstitute lyophilized antibodies?
2025-01-17 What information to look for before purchasing an antibody?
2024-12-30 Membrane activation before protein transfer: which one to use, methanol or ethanol?
2024-12-23 Comparing antibodies between each other, why does it not work?
2024-11-25 How to remove cross-reactivity to Rubisco of a primary antibody?
2024-09-24 Which is an optimal buffer for protein extraction?
2024-07-29
Archive
- April - 2025
- March - 2025
- February - 2025
- January - 2025
- December - 2024
- November - 2024
- September - 2024
- July - 2024
- June - 2024
- May - 2024
- March - 2024
- February - 2024
- December - 2023
- November - 2023
- September - 2023
- July - 2023
- May - 2023
- March - 2023
- January - 2023
- December - 2022
- November - 2022
- October - 2022
- September - 2022
- August - 2022
- June - 2022
- May - 2022
- March - 2022
- February - 2022
- January - 2022
- November - 2021
- October - 2021
- August - 2021
- June - 2021
- May - 2021
- April - 2021
- March - 2021
- February - 2021
- January - 2021
- December - 2020
- November - 2020
- October - 2020
- September - 2020
- August - 2020
- July - 2020
- June - 2020
- May - 2020
- April - 2020
- March - 2020
- January - 2020
- November - 2019
- October - 2019
- March - 2019
- April - 2017
- February - 2017
- May - 2016
- February - 2014
- September - 2013
- December - 2010
