
For Western blot:
Pre-immune serum dilution: 1:5000
Blocking: 5 - 10 % non-fat milk
Secondary antibody dilution: 1:25 000, 1h/RT (Agrisera goat anti-rabbit HPR conjugated, AS09 602)
Pre-immune serum screening is of crucial importantce before starting custom antibody production. If the aim is to use the produced antibody in a Western blot technique, pre-immune serum, which is serum collected from unimmunized animals, should be checked using this technique. If the technique used is immunolocalization, the serum should be tested as shown in the example below.
Please note that dilution of a serum is much higher for Western blot technique, usually 1: 5000 compare to immunlocalization.
Some animals may have developed antibodies contributing to background signals on the analysed material before immunization has even started. This test will help to determine which animals are suitable to be chosen for immunization, with the criteria that if there are any background singals, those bands should not be in close proximity of the MW of the target protein that is to be detected with the produced antibody.
In the example above, serum from 10 non-immunized animals were tested on samples from Arabidopsis thaliana (1) and Hordeum vulgare (2). In many samples, a prominent band around 55 kDa can be seen, and is most likely Rubisco. Some animals, like Animal 4, developed very distinct antibodies to a protein with MW 40 kDa and this animal should thus not be used to develop antibodies to a protein of 35, 45 or 50 kDa.
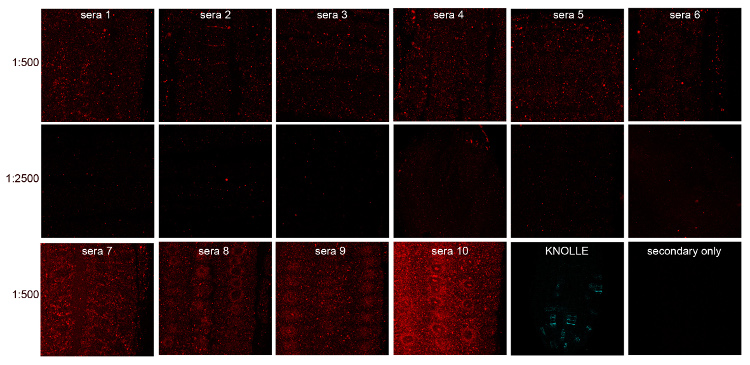
In the above example, serum collected from 10 different not immunized rabbits (pre-immune serum) were screened by immunofluorescence of 5-day-old roots of Arabidopsis thaliana, ecotype Col-0 (WT).
For Immunolocalization:
Pre-immune serum dilution: 1:500,
Secondary antibody dilution: 1:2500,
CY5-coupled donkey anti-rabbbit dilution: 1:300,
Positive control: polyclonal antibody, rabbit anti-KNOLLE at a dilution of 1:4000 (Lauber MH et al. 1997. J Cell Biol 139: 1485–1493),
Negative control: incubation with secondary antibody without primary antibody.
Courtesy of Dr. Anna Gustavsson and Prof. Markus Grebe, Umeå Plant Science Centre, Sweden.
Antibody production and validation process is outlined in the Agrisera Antibody Production Poster, which can be viewed here.
