1

Anti-Lci5 | low carbon dioxide induced protein number 5
AS05 090 | Clonality: Polyclonal | Host: Hen | Reactivity: Chlamydomonas reinhardtii
Limited stock
- Product Info
-
Immunogen: KLH-comjugated synthetic peptide chosen from a sequence of Chlamydomonas reinhardtii Lci5 protein A8IGD9
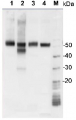
Host: Chicken Clonality: Polyclonal Purity: Immunogen affinity purified IgY in PBS pH 8.5. Contains 0.02% sodium azide. Format: Liquid Quantity: 100 µl Storage: Store at 4°C; make aliquots to avoid working with a stock. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Immunohistochemistry (IHC), Western blot (WB) Recommended dilution: 1 : 2000-1 : 5000 (WB) Expected | apparent MW: 32.7 | 24.1 kDa - Reactivity
-
Confirmed reactivity: Chlamydomonas reinhardtii Predicted reactivity: Chlamydomonas reinhardtii Not reactive in: No confirmed exceptions from predicted reactivity are currently known - Additional Information
-
Additional information: Antibody concentration is 0.9 µg/µl Additional information (application): First 180 amino acids of Lci5 are also found in EPYC1 protein of Chlamydomonas reinhardtii. This antibody will also recognize EPYC1 protein. - Background
-
Background: Lci5 protein is encoded by the nuclear gene lci5, which is induced during the acclimation to low CO2 conditions in the green alga Chlamydomonas reinhardtii. The Lci5 protein is peripherally associated with the stroma side of thylakoid membranes. In addition, this protein is heavily phosphorylated (7 sites, 3 Threonine and 4 serine residues, as shown by mass spec anlaysis) in cells growing under low CO2 concentrations in the medium or during state transitions (state 2). Microarray analysis showed that the gene is up-regulated 3 times during the acclimation to low CO2 conditions.
- Product Citations
-
Selected references: Turkina et al. (2006). CO2 limitation induces specific redox-dependent protein phosphorylation in Chlamydomonas reinhardtii. Proteomics 6:2693-26704. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extractsWestern Blot using IgY
Important note: Please, be aware, that often protocols used for IgG antibodies (rabbit, goat, mice) have to be optimised to get best results with IgY antibodies.1. Block unspecific binding by washing membrane in 25 ml blocking buffer/13x13 cm filer for 2 hours at room temperature on a shaker.
* Example of a Blocking buffer (Blotto)
1x TBS (20 mM Tris/HCl, pH 7.5,150 mM NaCl, 3-5 % low-fat dried milk powder, 0.05 % Tween-20 (or Nonidet P-40)
Blotto - Limitations:
o Usually very effective however: there are complex carbohydrates in milk and these will absorb out antibodies that recognize carbohydrate determinants.
o Some IgY antibodies might recognize milk proteins (high background signal).
o 10 % nonfat dry milk might block so efficiently, and in some cases so well, that no bands of interest will be seen.
Blocking insufficient - alternatives to try:Increase non-fat milk to 10-15 % 1h/RT incubation.
2. Wash each filter briefly, twice, then 1 x 15 minutes and 4 x 5 minutes at RT in Washing buffer. In case of still high background siganl - increase the length of a washing step even more than what is recommended above.
o Washing buffer:
1 x TBS, 0.05 % Tween-203. Add primary antibody to 10-20 ml of Antibody buffer (dilution range from 1:100 to 1: 30 000) and incubate the filter for 1-3 hours at room temperature (or 37°C, optional).
o Antibody buffer: 1 x TBS, 2% milk powder, 0.05 % Tween 204. Rinse the filter briefly twice, following by 1 x 15 and 4 x 5 minutes at RT in Washing buffer.
5. Add secondary antibody in Antibody Buffer. Use for instance rabbit or goat anti-IgY HRP conjugated. Start with the dilution at least 1 :10 000 (even higher dilutions are recommended).
Cross-reactivity signal coming from a secondary antibody can be easily checked by omitting a primary antibody in the whole procedure. Recommended secondary antibody from Agrisera: Rabbit anti-chicken HRP conjugated or Goat anti-chicken HRP conjugated or Goat anti-chicken ALP conjugated6. Wash the filter 4 x 5 minutes in Washing buffer, followed by 1 x 15 minutes in dH2O at room temperature.
Important!
or contact Agrisera Technical Support or talk with us directly on the website (bottom right corner).
Amount of membrane washes. In case of high unspecific background, increase the time of the wash with frequent exchange of Washing buffer.
In case of high background levels, firstly test if the secondary antibody is not contributing to the background. Use a small piece of empty transfer membrane, follow the procedure above without the step with primary antibodies. Recommended chemiluminescence detection reagent in mid picogram range: AgriseraECLBright, since primary antibodies can be used in a very high dilution, often background signal will be diluted out at that step.
Using extra sensitivite development systems (above extreme femtogram) can contribute to increased background signals.
Check also: Western Blot troubleshootingImmunoprecipitation using IgY
Important note: IgY DOES NOT BIND TO neither Protein G nor Protein A, therefore other methods should be considered as:
* Method 1:
Couple IgY antibodies to the matrix/beads.A. via free amino groups NHS-activated Sepharose Amersham Biosciences Technical Support or using AffiGel 15 BioRad
Coupling is done by free amino groups, therefore a possibility that antibody will be coupled via antigen binding domain must be considered.B. via sugar groups
using CarboLink Pierce or any other suitable for this purpose.
IgY is more heavily glycosilated than IgG in the Fc part of the antibody, this allows coupling to be matrix which will not interfeere with antigen binding domain.C. Dynal tosyl-activated magnetic beads (Invitrogen)
Afterwards follow standard immunoprecipitation protocol used for IgG.
Advantage of this method:
Heavy chain of IgY is ~ 70 [kDa]. This will allow to visualize smaller antigens on Western Blot without masking by heavy chains of IgY immunoglobulin, compare to use of IgG where heavy chain is around 50 [kDa].* Method 2:
Using a secondary anti-chicken IgY antibodies from rabbit or goat which are binding to Protein G or Protein A.General considerations:
* Optimization of the amount of precipitating antibody (IgY): Amount of IgY antibodies which should be used in the assay needs to be determined empirically. To begin with try similar concentration as the one used in a Western Blot.
* How to isolate a complex between antigen of interest and IgY antibodies? (IgY antibodies will not bind to neither Protein G nor Protein A)
Using anti-total IgY secondary antibodies (goat, rabbit) conjugated to Protein G (or Protein A)
* Consider:
Level of expression of your target protein present in cell lysate. Is it enough?
* Note:
Amount of the secondary, goat anti-chicken antibody:
Should be usually in 2-5 fold excess over the concentration of chicken antibody, to be sure that all of the chicken antibody-antigen complex is precipitated. 1 ml of the Protein G Sepharose has a capacity to bind 100 femtomoles of goat antibody.
* Avoid:
Presence of DTT or other reducing agents in the sample lysate, since they will distroy antibody. Extreme pH as well as the excess of detergent might also contribute to loose of the antibody activity.Examples of steps in one of immunoprecipitation protocols using IgY:
o First step
o Incubation of a cell supernatant with IgY antibodies Incubation over night, 4?C In some cases up to 1.5 M NaCl has been used in this step. Check if your target protein will cope with conditions. Use of PEG 6000 at 2 % can be also recommended to aid immunoprecipitation. Second step
o Adding goat anti-chicken antibody Incubation 2-4 h on ice Third step
Adding Protein G Sepharose Incubation by gentle rotation up 30-60 min. at 4?C (rocking might be less efficient).References where IgY has been used successfully in immunoprecipitation:
G-J. Lemamy et al. 1999 "High affinity antibodies from hen's-egg yolks against human mannose-6-phosphate/insulin-like growth-factor-II receptor (M6/IGFII-R): characterization and potential use in clinical cancer studies" Int. J. Cancer: 80, 896-902.
G. Camenisch et al. 1999 "General applicability of chicken egg yolk antibodies: the performance of IgY immunoglobulins raised against hypoxia-inducible factor 1 alfa" FASEB J: 13, 81-88 - Reviews:
-
This product doesn't have any reviews.