1
Anti-APX | L-ascorbate peroxidase
AS08 368 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. thaliana, A.maritima, Brassica sp., C. annuum, Citrus sp., D. muscipula, D. sanguinalis, E. crus-galli, l. formosana, L. sativus, M. esculenta, M. sativa, N. tabacum, O. sativa, P. miliaceum, P. zeylanica, S. lycopersicum, S. oleracea, S. superba, S. vulgaris, Triticum aestivum, Z. mays
- Product Info
-
Immunogen: BSA-conjugated synthetic peptide derived from Arabidopsis thaliana tAPX (thylakoidal ascorbate peroxidase) UniProt: Q42593-1, TAIR:At1g77490 and sAPX (stromal/mitochondrial ascorbate peroxidase) UniProt: Q42592-1 TAIR: At4g08390
Five out of twelve amino acids are also identical with cAPX1 (At1g07890), cAPX2 (At3g09640) and pAPX (At4g35000)Host: Rabbit Clonality: Polyclonal Purity: Serum Format: Lyophilized Quantity: 50 µl Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Western blot (WB) Recommended dilution: 1 : 2000 (WB) Expected | apparent MW: 25-38 kDa for A. thaliana
- Reactivity
-
Confirmed reactivity: Arabidopsis thaliana, Armeria maritima, Brassica napus, Capsicum annuum, Citrus sp., Digitaria sanguinalis, Dionaea muscipula, Echinochla crus-galli, Iris pumila, Lathyrus sativus, Liquidambar formosana, Lupin sp., Manihot esculenta, Medicago sativa, Nicotiana tabacum thylakoid-bound APX, stromal APX; Oryza sativa, Panicum milaceum, Plumbago zeylanica, Schima superba, Silene vulgaris, Solanum lycopersicum, Spinacia oleracea (stromal APX, thylakoid-bound), Triticum aestivum Predicted reactivity: Brassica rapa subsp. oleifera Stromal APX; Glycine max, Glycine soja L-ascorbate peroxidase T, chloroplastic; Medicago truncatula thylakoid-bound APX; Mesembryanthemum crystallinum, Pisum sativum Chloroplast stromal ascorbate peroxidase 12; Solanum lycopersicum thylakoid-bound APX; Spinacia oleracea stromal APX; Theobroma cacao L-APX T isoform 3; Vitis vinifera
Species of your interest not listed? Contact usNot reactive in: Algae, Helianthus annus, Marchantia polymorpha, - Application Examples
-
Application example

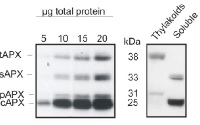
5 to 20 μg of total leaf protein from Arabidopsis thaliana (left panel) and chloroplast fractions (thylakoids and soluble, right panel) was separated on 15% polyacrylamide gel with 6M urea and blotted on PVDF. Filters were blocked 1h with 5% BSA, incubated with anti-APX antibody (1: 2000, 1h) followed by incubation with secondary HRP-coupled anti rabbit antibody (1: 10 000, 1h). Signal was detected with chemiluminescence detection reagent. AS08 368 is reactive to thylakoid (tAPX, 38 kDa), stromal (sAPX, 33 kDa), peroxisomal (pAPX, 31 kDa) and cytoplasmic (cAPX1 + cAPX2, 25 kDa) forms of ascorbate peroxidases.

Total proteins of Arabidopsis thaliana leaves were extracted with 10 % TCA and precipitated. The pellet was washed with acetone and resuspended in 100mM Tris-HCl (pH 7.5), 1mM EDTA, 2% (w= v) SDS, 1:100 of protease inhibitor cocktail (Thermo Scientific), 1 mM PMSF. Leaves were also grinded in 100 mM Tris-HCl (pH 7.5), MgCl2 10 mM, 1 mM EDTA, 1 mM PMSF, 1/100 of protease inhibitor cocktail and centrifugated. The supernatant (soluble fraction) was separated and the pellet (membrane fraction) was resuspended in the same buffer with 6 M urea and 1% SDS. Different amounts of proteins were separated in 15 % polyacrylamide gel with 6M urea after denaturation (70°C 5 min) and blotted on PVDF. Filters were blocked 1h with 5% BSA, Incubated with anti-APX antibodies at a dilution 1:2000, 1h/RT, washed 4 times with TBS tween (5 min each) and incubated with HRP coupled anti-rabbit IgG secondary antibody in dilution 1:10 000 1h/RT (AS09 602, Agrisera). After incubation with secondary antibody, the filter was washed 4 times with TBS (5 min each) and signal was detected with chemiluminescent detection reagent (30 secs exposition in film).
Courtesy Manuel Guinea Diaz, University of Turku, FinlandApplication examples: 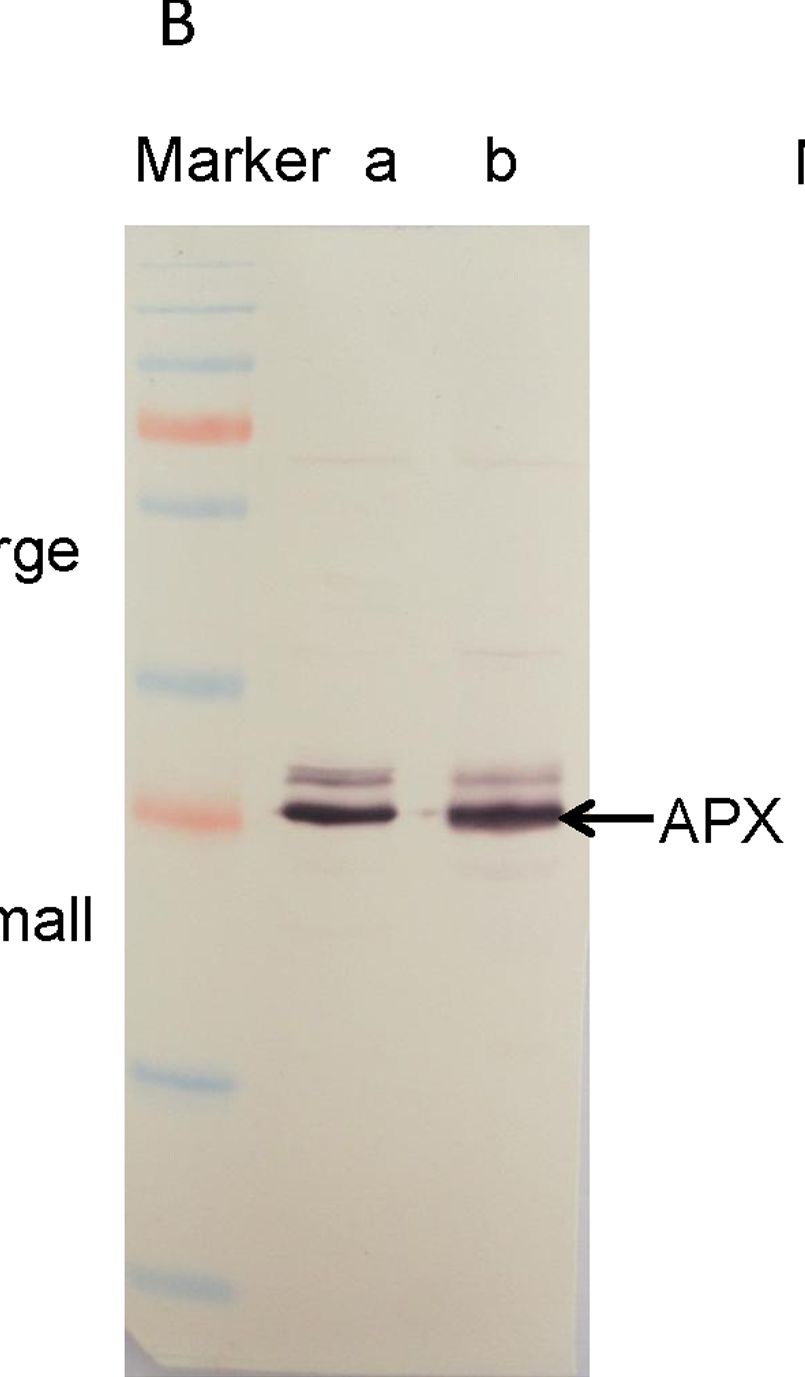
Reactant: Manihot esculenta (Cassava)
Application: Western Blotting
Pudmed ID: 24727655
Journal: PLoS One
Figure Number: 5B
Published Date: 2014-04-15
First Author: An, F., Fan, J., et al.
Impact Factor: 2.942
Open PublicationWestern blotting of Rubisco, APX and PrxQ.The expression of Rubisco, APX and PrxQ in leaves of cassava NZ199 diploid (a) and autotetraploid (b) genotypes were detected by western blotting using antiRubisco-polyclonal antibody (AS07218), anti-APX antibody (AS08368) and anti-PrxQ antibody (AS05093) from Agrisera, respectively.
Reactant: Liquidambar formosana (Chinese sweet gum)
Application: Western Blotting
Pudmed ID: 25025692
Journal: PLoS One
Figure Number: 6A
Published Date: 2014-07-16
First Author: Chen, J., Hu, W. J., et al.
Impact Factor: 2.942
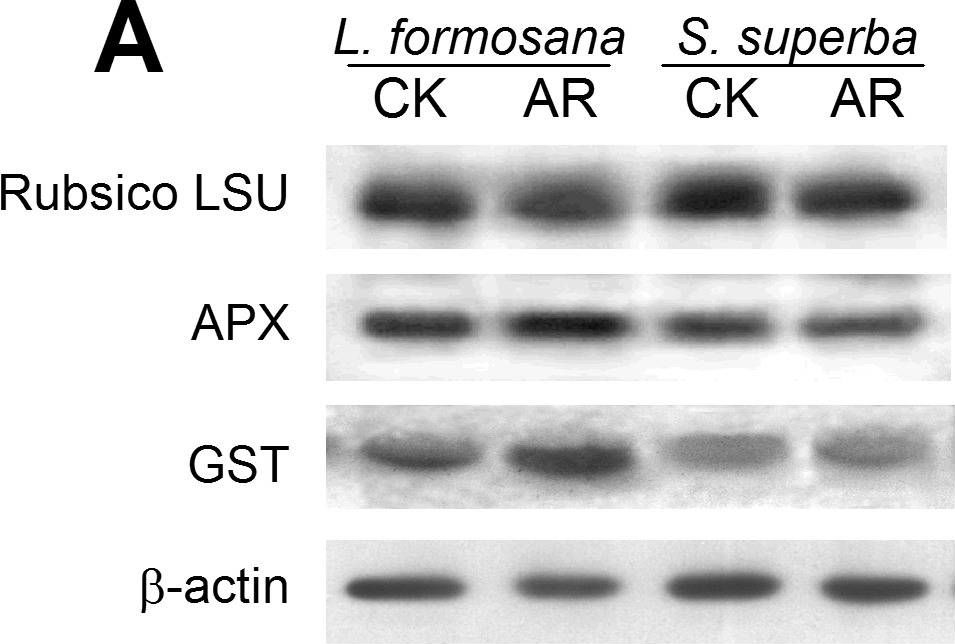
Open PublicationWestern blot analysis showing the expression of three protein spots.(A) Expression of rubulose-1,5-bisphoshate carboxylase large subunit (RuBisco LSU), ascorbate peroxidase (APX) and glutathione-S-transferase (GST) in L. formosana and S. superba seedlings after AR treatment. Relative expression level of RuBisco LSU (B), APX (C) and GST (D) were analyzed with the Quantity One software. ?-actin was used as the internal control. Means with different letters indicate significantly difference (p<0.05) with regard to AR treatments.
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 30609769
Journal: Int J Mol Sci
Figure Number: 4E
Published Date: 2019-01-02
First Author: Szyma?ska, K. P., Polkowska-Kowalczyk, L., et al.
Impact Factor: 5.542
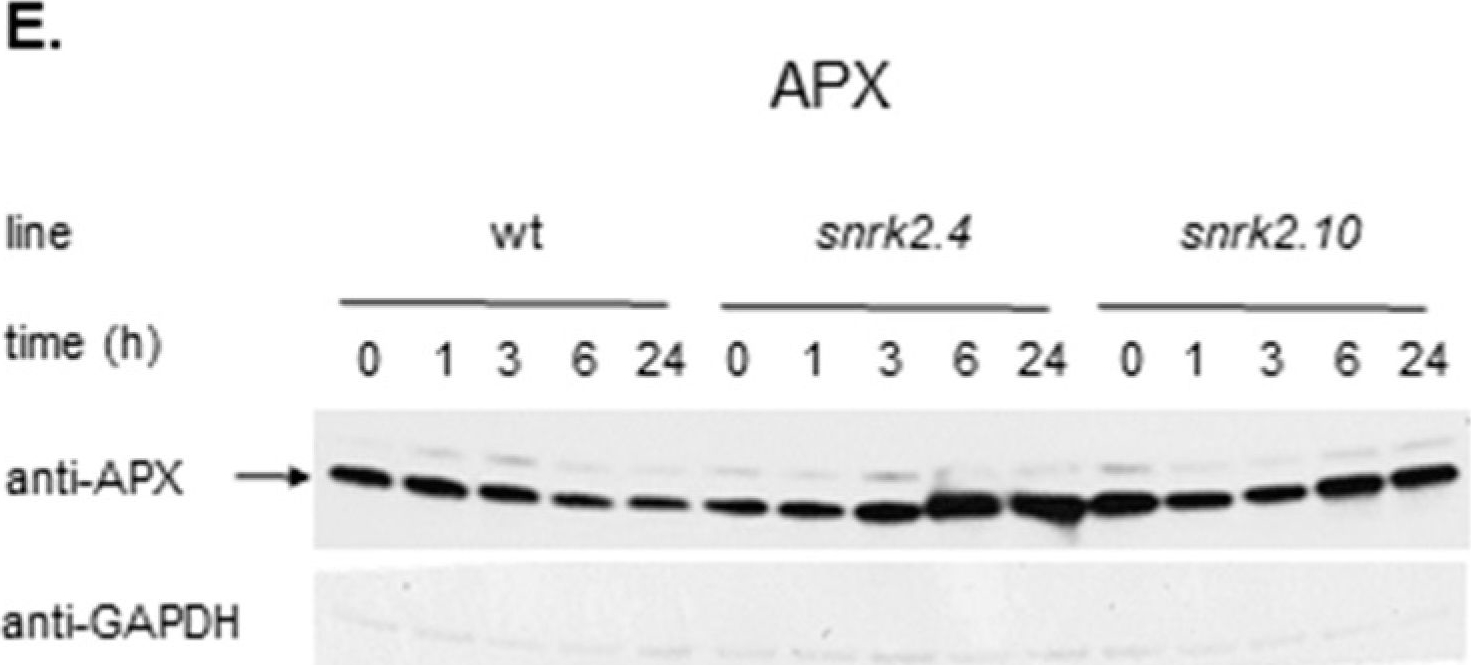
Open PublicationSnRK2.4 and SnRK2.10 regulate enzymes of the ascorbate cycle during response to salt stress. Wild type and snrk2 mutant seedlings were subjected to treatment with 150 mM NaCl for times indicated. Expression of (A) APX1—Ascorbate Peroxidase 1, (B) APX2—Ascorbate Peroxidase 2, (C) APX6—Ascorbate Peroxidase 6, and (D) DHAR1—Dehydroascorbate Reductase 1 was monitored by RT-qPCR; error bars represent SD; stars represent statistically significant differences in comparison with the wt plants (Student t-test) where * p < 0.05; ** p < 0.001; *** p < 0.0001. Total protein level of (E) APX and (F) DHAR1 was monitored with immunoblot analysis; after exposure, membranes were stripped and reused for GAPDH detection as a loading control. At least two independent biological replicates of the experiment were performed. Results of one representative experiment are shown.
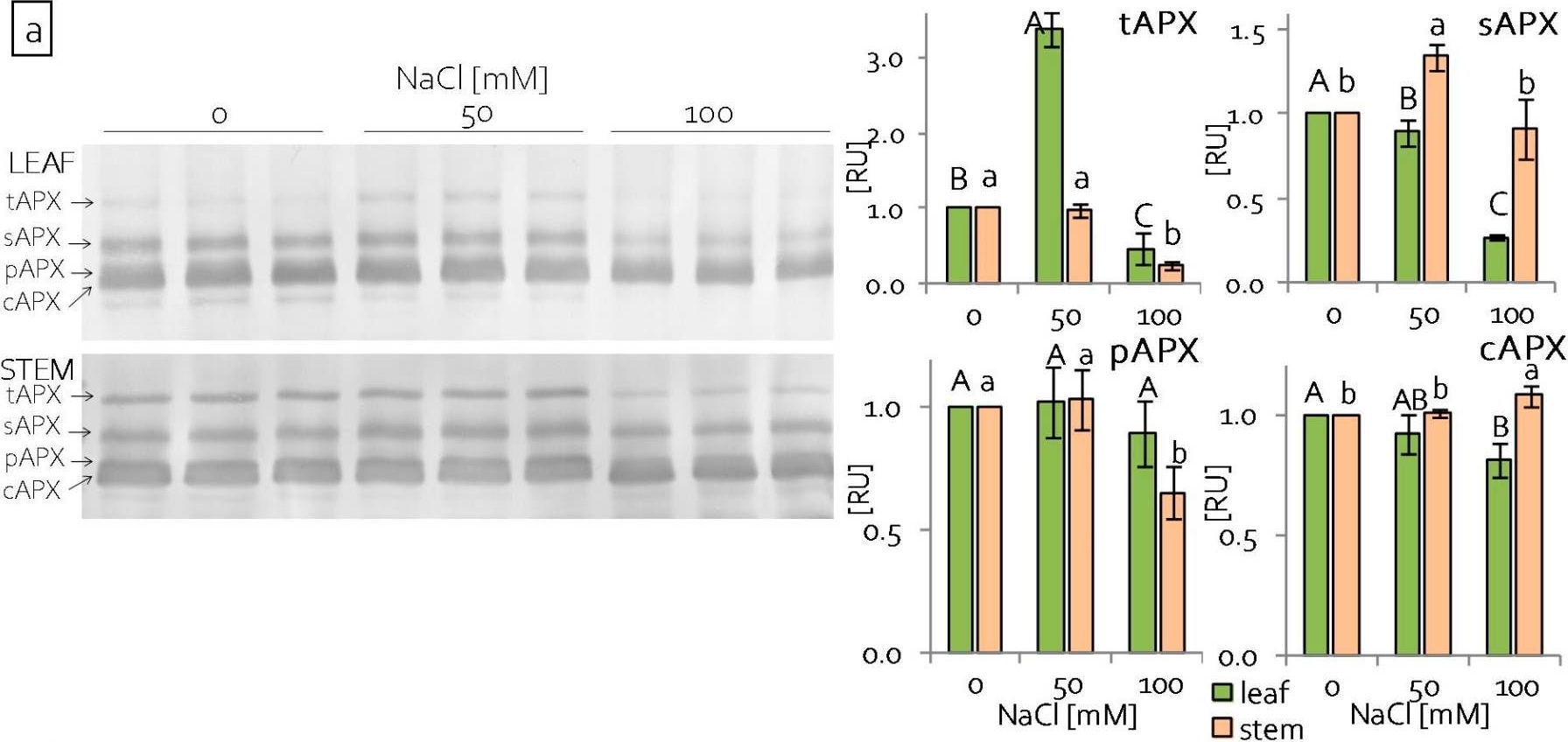
Reactant: Pisum sativum (Pea)
Application: Western Blotting
Pudmed ID: 33445673
Journal: Int J Mol Sci
Figure Number: 3A
Published Date: 2021-01-12
First Author: Tokarz, K. M., Weso?owski, W., et al.
Impact Factor: 5.542
Open PublicationContent of antioxidant enzymes: (a) APX isoforms and (b) CAT in leaves and stems of grass pea shoots under salinity; tAPX—thylakoid ascorbate peroxidase, sAPX—stromal ascorbate peroxidase, pAPX—peroxisomal ascorbate peroxidase, cAPX—cytoplasmic ascorbate peroxidise, CAT—catalase; content of proteins expressed as relative units [RU]; different letters—statistically significant differences within each organ (leaf-uppercase, stem-lowercase) at p ? 0.05 (n = 3).
- Additional Information
-
Additional information (application): This product can be sold containing proclin if requested
- Background
-
Background: APX plays a key role in plant antioxidant system by reducing hydrogen peroxide to water. Cellular localization includes chloroplast (tAPX and sAPX), cytosol (cAPX) and peroxisome (pAPX).
- Product Citations
-
Selected references: Kolbert et al. (2023). Nitro-oxidative response to internalized multi-walled carbon nanotubes in Brassica napus and Solanum lycopersicum. Ecotoxicol Environ Saf. 2023 Nov 15:267:115633. doi: 10.1016/j.ecoenv.2023.115633.
Bismarck et al. (2023). Growth in fluctuating light buffers plants against photorespiratory perturbations. Nat Commun . 2023 Nov 3;14(1):7052. doi: 10.1038/s41467-023-42648-x.
Burian et al (2023) A prospective study of short-term apoplastic responses to ammonium treatment. J Plant Physiol. 2023 Jul;286:154008. doi: 10.1016/j.jplph.2023.154008.
Fortunato et al. (2022) GUN1 involvement in the redox changes occurring during biogenic retrograde signaling. Plant Science. Volume 320, July 2022, 111265, https://doi.org/10.1016/j.plantsci.2022.111265
Borysiuk et al. (2022) Glyoxalase I activity affects Arabidopsis sensitivity to ammonium nutrition. Plant Cell Rep. 2022;41(12):2393-2413. doi:10.1007/s00299-022-02931-5
Miernicka et al. (2022) The Adjustment Strategy of Venus Flytrap Photosynthetic Apparatus to UV-A Radiation. Cells. 2022;11(19):3030. Published 2022 Sep 27. doi:10.3390/cells11193030
Molnar et al. (2022) Limited Zn supply affects nutrient distribution, carbon metabolism and causes nitro-oxidative stress in sensitive Brassica napus, Environmental and Experimental Botany,Volume 202, 2022, 105032,ISSN 0098-8472,https://doi.org/10.1016/j.envexpbot.2022.105032.
Seiml-Buchinger et al. (2022) Ascorbate peroxidase postcold regulation of chloroplast NADPH dehydrogenase activity controls cold memory. Plant Physiol. 2022;190(3):1997-2016. doi:10.1093/plphys/kiac356
Wang et al. (2022) Reciprocity between a retrograde signal and a putative metalloprotease reconfigures plastidial metabolic and structural states. Sci Adv. 2022 Jun 3;8(22):eabo0724. doi: 10.1126/sciadv.abo0724. Epub 2022 Jun 3. PMID: 35658042; PMCID: PMC9166295.
Kucko et al. (2022) The acceleration of yellow lupine flower abscission by jasmonates is accompanied by lipid-related events in abscission zone cells, Plant Science, Volume 316, 2022,111173, ISSN 0168-9452, https://doi.org/10.1016/j.plantsci.2021.111173. (https://www.sciencedirect.com/science/article/pii/S0168945221003691)
Jedelska et al. (2021) Protein S-nitrosation differentially modulates tomato responses to infection by hemi-biotrophic oomycetes of Phytophthora spp. Hortic Res. 2021 Feb 1;8(1):34. doi: 10.1038/s41438-021-00469-3. PMID: 33518717; PMCID: PMC7848004.
Tokarz et al. (2021). Stem Photosynthesis-A Key Element of Grass Pea (Lathyrus sativus L.) Acclimatisation to Salinity. Int J Mol Sci. 2021 Jan 12;22(2):685. doi: 10.3390/ijms22020685. PMID: 33445673; PMCID: PMC7828162.
Tokarz et al. (2020). Can Ceylon Leadwort ( Plumbago zeylanica L.) Acclimate to Lead Toxicity?-Studies of Photosynthetic Apparatus Efficiency. Int J Mol Sci. 2020 Mar 9;21(5):1866.doi: 10.3390/ijms21051866.
Molnar et al. (2020). Nitro-oxidative Signalling Induced by Chemically Synthetized Zinc Oxide Nanoparticles (ZnO NPs) in Brassica Species. Chemosphere, 251, 126419
Jedelska et al. (2019). Tomato Root Growth Inhibition by Salinity and Cadmium Is Mediated By S-Nitrosative Modifications of ROS Metabolic Enzymes Controlled by S-Nitrosoglutathione Reductase. Biomolecules. 2019 Aug 21;9(9). pii: E393. doi: 10.3390/biom9090393.
Szymanska et al. (2019). SNF1-Related Protein Kinases SnRK2.4 and SnRK2.10 Modulate ROS Homeostasis in Plant Response to Salt Stress. Int J Mol Sci. 2019 Jan 2;20(1). pii: E143. doi: 10.3390/ijms20010143.
Deng et al. (2019). Integrated proteome analyses of wheat glume and awn reveal central drought response proteins under water deficit conditions. J Plant Physiol. 2019 Jan;232:270-283. doi: 10.1016/j.jplph.2018.11.011.
Balfagon et al. (2018). Involvement of ascorbate peroxidase and heat shock proteins on citrus tolerance to combined conditions of drought and high temperatures. Plant Physiol Biochem. 2018
Cunha et al. (2016). Salinity and osmotic stress trigger different antioxidant responses related to cytosolic ascorbate peroxidase knockdown in rice roots. Environmental and Experimental Botany, Volume 131, November 2016, Pages 58-67.
Ko et al. (2016). Constitutive expression of a fungus-inducible carboxylesterase improves disease resistance in transgenic pepper plants. Planta. 2016 Aug; 244(2):379-92. doi: 10.1007/s00425-016-2514-6. Epub 2016 Apr 13.
Yin et al. (2016). Comprehensive Mitochondrial Metabolic Shift during the Critical Node of Seed Ageing in Rice. PLoS One. 2016 Apr 28;11(4):e0148013. doi: 10.1371/journal.pone.0148013. eCollection 2016.
Vuleta et al. (2016). Adaptive flexibility of enzymatic antioxidants SOD, APX and CAT to high light stress: The clonal perennial monocot Iris pumila as a study case. Plant Physiol Biochem. 2016 Mar;100:166-73. doi: 10.1016/j.plaphy.2016.01.011. Epub 2016 Jan 19
Hattab et al. (2015). Characterisation of lead-induced stress molecular biomarkers in Medicago sativa plants. Environm. Exp. Botany. Volume 123, March 2016, Pages 1–12.
Parys et al. (2014). Metabolic Responses to Lead of Metallicolous and Nonmetallicolous Populations of Armeria maritima. Arch Environ Contam Toxicol. 2014 Jul 29.
Feifei et al. (2014). Comparison of Leaf Proteomes of Cassava (Manihot esculenta Crantz) Cultivar NZ199 Diploid and Autotetraploid Genotypes. PLoS One. 2014 Apr 11;9(4):e85991. doi: 10.1371/journal.pone.0085991. eCollection 2014.
Sobrino-Plata et al. (2014). Glutathione is a key antioxidant metabolite to cope with mercury and cadmium stress. Plant Soil, DOI 10.1007/s11104-013-2006-4. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Oxygenic photosynthesis poster by prof. Govindjee and Dr. Shevela
Z-scheme of photosynthetic electron transport by prof. Govindjee and Dr. Björn and Dr. Shevela - Reviews:
-
MIO | 2015-02-20We use Arabidopsis thaliana whole leaf as sample. We did Western blot 1:2000 works wonderful!Elzbieta Romanowska | 2013-10-31We used this antibody for APX immunodetection analysis (ECL analysis) in the leaves of different subtypes of C4 plants. SDS-PAGE was performed on 15% polyacrylamide gel and 7 ug protein was loaded to each lane. We used 1:2000 dilution. The highest signal was observed in Panicum milliaceum (NAD-ME subtype), Zea mays (NADP-ME subtype) and Echinochloa cruss- galli (NADP-ME). The weakest signal was noted in Digitaria sanguinalis (NADP-ME), but this may be caused by different protein amount in the given conditions.Anna | 2013-10-14I used this antybody on leaf and mitochondrial extracts and it perfectly worked for both.details: Arabidopsis thaliana, 20ug leaf extract, 10ug mitochondrial extract per lane, 1:2000 dilution, ECL detection.| 2011-10-04We tried with success this antibody in Medicago sativa, with 10 ug protein loading, with a 1/2000 dilution of primary antibody and 1/20000 secondary HRP, suitable for ECL detection
Accessories
AS06 180 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. thaliana, A. toxicaria, H.vulgare, M. sativa, O. satica, P.sativum, S. lycopersicum, S. tuberosum, S. vulgaris, P. silvestris, . Z. mays
Replaced by AS23 4940


