1
Anti-cAPX | Ascorbate peroxidase (cytosolic) (plant)
AS06 180 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. thaliana, A. toxicaria, H.vulgare, M. sativa, O. satica, P.sativum, S. lycopersicum, S. tuberosum, S. vulgaris, P. silvestris, . Z. mays
Replaced by AS23 4940
- Product Info
-
Immunogen: KLH-conjugated peptide derived from N-terminal of Zea mays cytosolic APX UniProt: Q41772
Host: Rabbit Clonality: Polyclonal Purity: Total IgG. Protein G purified in PBS pH 7.4. Format: Lyophilized Quantity: 100 µl Reconstitution: For reconstitution add 100 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Immunoprecipitation (IP), Immunolocalization (IL), Western blot (WB) Recommended dilution: 2 µg (IP), 1 : 500 (IL), 1: 2000 - 1 : 10 000 (WB) Expected | apparent MW: 28 kDa
- Reactivity
-
Confirmed reactivity: Arabidopsis thaliana, A. toxicaria, Hordeum vulgare, Medicago sativa, Nicotiana tabacum, Oryza sativa, Pisum sativum, Salicornia sp., Silene vulgaris, Solanum lycopersicum, Solanum lycopersicum, Solanum tuberosum, Picea silvestris, Zea mays, Zygophyllum fabago
Predicted reactivity: Brassica. juncea, Citrus sinensis, Fragaria ananassa, Gossypium hirsutum, Solanum tuberosum, Sorghum bicolor, Pinus pinaster, Vitis vinifera
Species of your interest not listed? Contact usNot reactive in: Algae, Galdieria sulphuraria, Glycine max - Application Examples
-
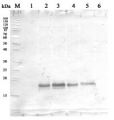
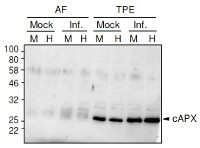
Application example 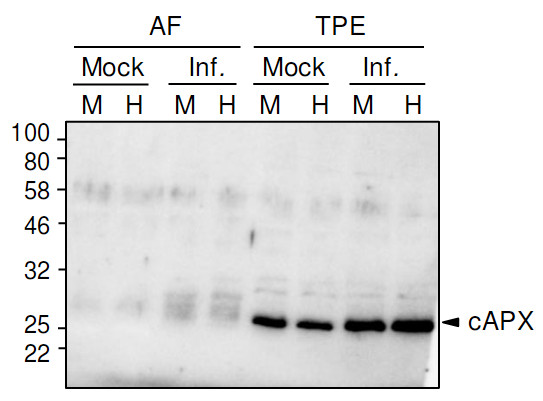
40 µg of total protein from Solanum lycopersicum extracted with Extraction buffer (1x Hepes buffer, cOmplete Mini protease inhibitor cocktail (1 tablet/10ml), 5mM DTT) and denatured with 1x SDS-loading buffer at 95°C for 5 min. Samples were separated on 12 % SDS-PAGE and blotted 1h to PVDF using dry transfer. Blots were blocked with 5% milk in TBST for 45 min with agitation. Blot was then washed once with TBST, and then incubated in the primary antibody a-cAPX at a dilution of 1:2000 ON/4°C with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 3 times for 5 min in TBS-T at RT with agitation. Blot was incubated in Agrisera matching secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, AS09 602) diluted to 1:5000 in for 30min/RT with agitation. The blot was washed as above and developed for 2 min with chemiluminescence detection reagent. Exposure time was set to automatic.
Courtesy Dr. Nuria Sanchez Coll, CRAG, Spain
20 µg of total protein from Arabidopsis thaliana total (1), soluble (2) and membrane (3) fractions were prepared according to Planas-Marquès et al. (2016) and denatured with 1x SDS-loading buffer at 95°C for 5 min. Samples were separated on 12 % SDS-PAGE and blotted 1h to PVDF using dry transfer. Blots were blocked with 12 % milk in TBST for 45 min with agitation. Blot was then washed once with TBST, and then incubated in the primary antibody a-cAPX at a dilution of 1:10 000 ON/4°C with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 3 times for 5 min in TBS-T at RT with agitation. Blot was incubated in Agrisera matching secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, AS09 602) diluted to 1:5000 in for 30min/RT with agitation. The blot was washed as above and developed with chemiluminescent detection reagent. Exposure time was set to automatic.
Courtesy Dr. Nuria Sanchez Coll, CRAG, Spain
(A) , (B)- control antibody, anti-PIN1, (C,D) immunolocalization using anti-cAPX antibodies.
Courtesy of Dr. Taras Pasternak; Freiburg University, Germany

Dissociated rat hippocampal neurons 14 DIV Transfection of FLAG- and Nuclear Export Signal-tagged APEX2 (variant of cAPX; FLAG-APEX2-NES). Fixation: 4% formaldehyde for 15 min RT, 0.1% TritonX-100 permeabilization 20 min RT, 5% FCS blocking 1 hr RT. All in PBS. Primary antibody: 1:200 rabbit anti-cAPX, 4°C incubated for 24h, 1:1000 mouse anti-FLAG, RT 1h.
Secondary antibodies: 1:1000 anti-rabbit Alexa488, RT 1 h, 1:1000 anti-mouse Alexa561, RT 1 h.
Courtesy of Dr. Tony Cijsouw, Biederer lab, Department of Neuroscience Tufts University School of Medicine, USA - Additional Information
-
Additional information: Total IgG concentration is 3 µg/µl - Background
-
Background: Ascorbate peroxidase (APX) is the enzyme catalyzing the ascorbate-dependent reduction of hydrogen peroxide. Ascorbate (AA) plays a key role in defense against oxidative stress and is particularly abundant in fruits and photosynthetic tissues. AA is found in every compartment of the plant cell including the apoplast.
- Product Citations
-
Selected references: Li et al. (2024). An NLR paralog Pit2 generated from tandem duplication of Pit1 fine-tunes Pit1 localization and function. Nat Commun. 2024 May 30;15(1):4610. doi: 10.1038/s41467-024-48943-5.
Flores-Cáceres et al. (2023). The Early Oxidative Stress Induced by Mercury and Cadmium Is Modulated by Ethylene in Medicago sativa Seedlings. Antioxidants 2023, 12, 551. https://doi.org/10.3390/antiox12030551
Bègue et al. (2019). CDC48 regulates ascorbate peroxidase in tobacco. J Exp Bot. 2019 Mar 1. pii: erz097. doi: 10.1093/jxb/erz097.
Wang et al. (2018). Resistance protein Pit interacts with the GEF OsSPK1 to activate OsRac1 and trigger rice immunity. Proc Natl Acad Sci U S A. 2018 Nov 16. pii: 201813058. doi: 10.1073/pnas.1813058115.
Balážová et al. (2018). Zinc oxide nanoparticles phytotoxicity on halophyte from genus Salicornia. Plant Physiol Biochem. 2018 Sep;130:30-42. doi: 10.1016/j.plaphy.2018.06.013.
Adhikari et al. (2018). Sulfate improves cadmium tolerance by limiting cadmium accumulation, modulation of sulfur metabolism and antioxidant defense system in maize. Environmental and Experimental Botany Volume 153, September 2018, Pages 143-162.
Ferrer et al. (2018). Differential Pb tolerance in metallicolous and non-metallicolous Zygophyllum fabago populations involves the strengthening of the antioxidative pathways. Environ & Exp Botany, Vo. 150, June 2018, Pages 141-151.
Aroca et al. (2015). S-sulfhydration: a new post-translational modification in plant systems. Plant Physiology March 2015 pp.00009.2015.
Terrile et al. (2014). Nitric oxide-mediated cell death is triggered by chitosan in F. eumartii spores. Pest Manag Sci. 2014 Apr 25. doi: 10.1002/ps.3814.
Tsaniklidis et al. (2013). L-Ascorbic acid metabolism in parthenocarpic and seeded cherry tomatoes. Plant Growth Regul,DOI 10.1007/s10725-013-9845-0. (Solanum lycopersicum, immunolocalization) - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts - Reviews:
-
Damián Balfagón | 2017-11-21I used it for Citrus sinensis. I had excellent resolution of the cAPX bands. It also appears the bands of tylAPX and stromal APX wich I had to diferenciate.