1
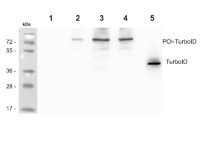
Anti-BirA (mutated/TurboID)
AS20 4440 | Clonality: Polyclonal | Host: Rabbit | Reactivity: E. coli BirA - mutated/TurboID overexpressed in various organisms
- Product Info
-
Immunogen: Recombinant mutated BirA protein from E.coli produced using the following plasmid: TurboID-His6_pET21a, (Plasmid #107177). Expression was done in a vector that allowed for the generation of an untagged protein (without HIS6tag). Host: Rabbit
Clonality: Polyclonal Purity: Immunogen affinity purified serum in PBS pH 7.4. Format: Lyophilized Quantity: 50 µg Reconstitution: For reconstitution add 50 µl, of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Immunofluorescence (IF), Western blot (WB) Recommended dilution: 1 : 5000 (WB) Expected | apparent MW: 35 kDa - Reactivity
-
Confirmed reactivity: BirA (mutated/TurboID) Predicted reactivity: mini TurboID Not reactive in: No confirmed exceptions from predicted reactivity are currently known - Application Examples
-

1 – 20 µg A. thaliana – Wt Col-0 (Negative control)
2 – 20 µg of A. thaliana expressing POI-TurboID fusion (Independent line 1)
3 – 20 µg of A. thaliana expressing POI-TurboID fusion (Independent line 2)
4 – 20 µg of A. thaliana expressing POI-TurboID fusion (Independent line 3)
5 – 10 ng of purified TurboID (Positive control)20 µg/well of total protein were extracted from Arabidopsis thaliana leaf material in diluted HENS (25mM HEPES pH 7.7, 1mM EDTA, 2.5 % SDS) and stored at -80°C. Samples were denatured in 1x protein loading dye (0.5% Sodium dodecyl Sulfate, 0.002% Bromophenol Blue, 10% glycerol, and 50 mM Tris-HCl pH6.8) at 95°C for 5 min. Samples were separated on 4-16% gradient SDS-PAGE gel and blotted 1h to a nitrocellulose membrane (pore size of 0.45 um), using a semi-dry transfer. Blot was blocked with 5% milk in TBS-T at 4°C/ON without agitation. Blot was incubated in the primary antibody at a dilution of 1:5000 in 5% milk in TBS-T, at 4°C, ON without agitation. The antibody solution was decanted and the blot was rinsed briefly, then washed three times for 10 min in TBS-T at RT with agitation. Blot was incubated in Agrisera matching secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, AS09 602) diluted to 1:25 000 in TBS-T for 1h at RT with agitation. The blot was washed as above and developed for 2 min with Agrisera ECLBright. Exposure time was 172 seconds.
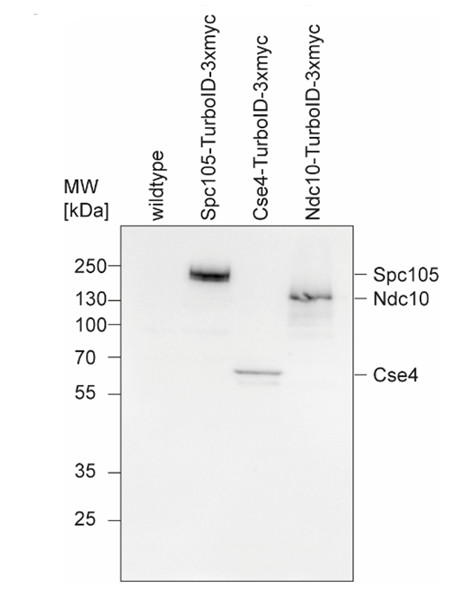
Samples:1 - wildtype strain DDY902
2 - Spc105-TurboID-3xmyc, MW: 135 kDa
3 - Ces4-TurboID-3xmyc, MW: 57 kDa
4 - Ndc10-TurboID-3xymyc, MW: 140 kDa
1 µg/well of total protein extracted freshly from Saccharomyces cerevisiae according to Kushnirov, 2000. Exact buffer components were: (6xLD = 300mM Tris pH6.8, 12% SDS, 60% Glycerol, 4% beta-Mercaptoethanol, Bromphenol blue) and denatured with 50µl 2x LD (in ddH2O diluted) at 95°C 5 min. Samples were separated on 10 % SDS-PAGE and blotted for 2 h, 400 mA constant to nitrocellulose (pore size of 0.45 µm), using: wet transfer in the cold. Blot was blocked with 5 % milk for: 1h/RT with agitation. Following membrane washes, the blot was incubated in the primary antibody at a dilution of 1:5 000 in 5% milk in TBST with agitation ON/4°C. The antibody solution was decanted, and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at RT with agitation. Blot was incubated in matching secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated) diluted to 1: 20 000 in 5% milk in TBST for 1 h/RT with agitation. The blot was washed as above and developed with a following chemiluminescent detection reagent. Exposure time was 2 minutes.Courtesy of Dr. Sonja Haberkorn, Molekulare Genetik, Universität Duisburg-Essen, Germany

1 – 1 ng of purified TurboID 2 – 5 ng of purified TurboID 3 – 10 ng of purified TurboID 4 – 25 ng of purified TurboID 5 – 50 ng of purified TurboID 6 – 75 ng of purified TurboID Mark: PageRulerTM Plus Prestained Protein Ladder; ThermoFisher Scientific; MW of TurboID = 35 kDa
1ng, 5 ng, 10 ng, 25 ng, 50 ng, and 75 ng loaded into wells 1, 2, 3, 4, 5, and 6, respectively, of purified TurboID in 1x Phosphate Buffer Saline (PBS) were stored at -80°C. Samples were denatured in 1x protein loading dye (0.5% Sodium dodecyl Sulfate, 0.002% Bromophenol Blue, 10% glycerol, and 50 mM Tris-HCl pH6.8) at 95°C for 5 min. Samples were separated on 4-16% gradient SDS-PAGE gel and blotted 1h to a nitrocellulose membrane (pore size of 0.45 um), using a semi-dry transfer. Blot was blocked with 5% milk in TBS-T at 4°C/ON without agitation. Blot was incubated in the primary antibody at a dilution of 1:1000 in 5% TBS-T, at 4°C, ON without agitation. The antibody solution was decanted and the blot was rinsed briefly, then washed three times for 10 min in TBS-T at RT with agitation. Blot was incubated in Agrisera matching secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, AS09 602) diluted to 1:25 000 in TBS-T for 1h at RT with agitation. The blot was washed as above and developed for 2 min with Agrisera ECLBright. Exposure time was 92 seconds.
Courtesy of Eli Gordon and Dr. Patrick Treffon, Elizabeth Vierling Lab Department of Biochemistry and Molecular Biology University of Massachusetts Amherst, USA - Background
-
Background: Tagging a protein with TurboID allows studying protein interactions in different types of cells, organs and developmental stages. This is a suitable tool for proximity labelling experiments as described in Mair et al. (2019). Proximity labelling of protein complexes and cell-type-specific organellar proteomes in Arabidopsis enabled by TurboID. Elife . 2019 Sep 19;8:e47864. doi: 10.7554/eLife.47864. - Product Citations
-
Selected references: Persyn et al. (2024).A Nitrogen-Specific Interactome Analysis Sheds Light on the Role of the SnRK1 and TOR Kinases in Plant Nitrogen Signaling. Mol Cell Proteomics. 100842. doi: 10.1016/j.mcpro.2024.100842.
Liu et al. (2024). Activation of kappa opioid receptor suppresses post-traumatic osteoarthritis via sequestering STAT3 on the plasma membrane. Cell Commun Signal. 2024 Jun 18;22(1):335.doi: 10.1186/s12964-024-01709-4.
Medica et al. (2023). Proximity-dependent mapping of the HCMV US28 interactome identifies RhoGEF signaling as a requirement for efficient viral reactivation. PLoS Pathog. 2023 Oct 2;19(10):e1011682.doi: 10.1371/journal.ppat.1011682.
Shi et al. (2023). Protocol to identify protein-protein interaction networks in Solanum tuberosum using transient TurboID-based proximity labeling. STAR Protoc. 2023 Sep 20;4(4):102577.doi: 10.1016/j.xpro.2023.102577. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Agrisera Educational Poster Collection - Reviews:
-
This product doesn't have any reviews.

