1
Anti-UCP | Uncoupling protein
AS12 1850 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. thaliana, G.max, S. lycopersicum, T. aestivum, V. faba
- Product Info
-
Immunogen: KLH-conjugated synthetic peptide derived from known UCP protein sequences, including UCP1 (AT3G54110) and UCP2 (AT5G58970) of Arabidopsis thaliana
Host: Rabbit Clonality: Polyclonal Purity: Immunogen affinity purified serum in PBS pH 7.4. Format: Lyophilized Quantity: 100 µg Reconstitution: For reconstitution add 100 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Immunolocalization (IL), Western blot (WB) Recommended dilution: 1: 200 (IL), 1 : 2000 (WB) Expected | apparent MW: 32 kDa
- Reactivity
-
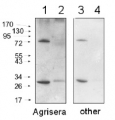
Confirmed reactivity: Arabidopsis thaliana, Splanum lycopersicum, Triticum aestivum, Vicia faba (protoplasts)
Predicted reactivity: Citrus sinensis, Dracunulus vulgaris, Glycine max, Litchi chinensis, Medicago tribuloides, Nannochloropsis gaditana, Nicotiana tabacum, Oryza sativa, Populus trichocarpa, Ricinus communis, Saccharum officinarium (sugarcane), Triticum aestivum
Species of your interest not listed? Contact usNot reactive in: No confirmed exceptions from predicted reactivity are currently known - Application Examples
-
Application example
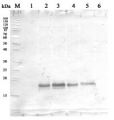
25 µg (1) or 50 µg (2) of mitochondria, isolated from 14-day old Col-0 plants (Arabidopsis thaliana) grown in hydroponic cultures, were separated on a 12.5% acrylamide-SDS-PAGE. The unstained peqGOLD low molecular weight (LMW) protein marker was used as a molecular weight standard. The gel was subsequently incubated in transfer buffer (40 mM glycine, 100 mM Tris, 0.375 % (w/v) SDS, pH 8.9-9) for 30 minutes at room temperature. Semidry western blotting was performed with 3 layers of whatman paper soaked in transfer buffer and blotted at 0.8 mA/ cm2 gel for one hour at room temperature. Blocking was performed for one hour in TBS-T (150 mM NaCl, 10 mM Tris pH 7.4, 0.1 % (v/v) Tween-20) with the addition of 3 % (w/v) non-fat milk at room temperature. Primary antibody (1:2000 dilution) incubation was performed at 4°C/ON in the presence of 1 % (w/v) non-fat milk in TBS-T. Secondary antibody goat anti-rabbit HRP conjugated (AS09 602 Agrisera), at 1:10 000 dilution) was incubated 1h/RT in TBS-T. Chemiluminescence was detected using a 1:1 ratio mixture of ECL 1 (100 mM Tris, 1 % (w/v) luminol, 0.44 % (w/v) coumaric acid, pH 8.5) and ECL 2 (100 mM Tris, 0.18 % (v/v) H2O2, pH 8.5) solution and visualized using an Image QuantLAS4000 image visualizer (GE healthcare). The membrane was exposed for 60 seconds.
UCP was detected at a size of approximately 29 kDa.
Courtesy Dr. Tamara Hechtl, Munich University, Germany
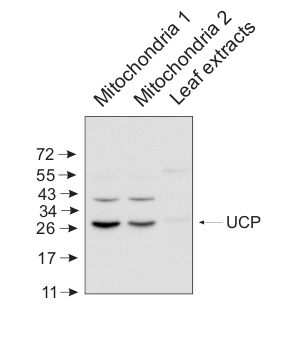
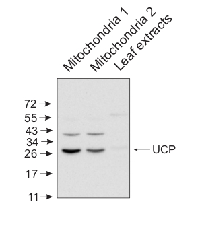
10 μg of mitochondrial fraction from Arabidopsis thaliana and 25 μg of Arabidopsis thaliana leaf extract were separated on 10% gel and blotted on nitrocellulose membrane using wet transfer (0.22% CAPS, pH 11). Filters where blocked (1.5h) in 5% milk in TBST (1X TBS, 0,1% Tween 20), incubated with 1: 5000 anti-UCP antibodies (2h in TBST) followed by incubation with 1: 10 000 secondary anti-rabbit (1h) HRP-coupled antibodies from Agrisera, AS09 602 and visualized with standard ECL on Kodak autoradiography film for 15-60 s. Mitochondria were isolated as described by Urantowka et al. (Plant Mol Biol, 2005, 59:239-52). Mitochondrial pellets were suspended in 1X Laemmli buffer (5% beta-mercaptoetanol, 3.7% glycerol, 1.1% SDS, 23 mM Tris- HCl pH 6.8, 0.01% bromophenol blue), heated (95°C, 5 min.) and centrifuged (13 000rpm, 1 min.). Leaf extracts were prepared as described by Martinez-Garcia et al. (Plant J., 1999, 20:251-7).Courtesy Dr. Janusz Piechota, Wrocław University, Poland - Additional Information
-
Additional information: Peptide used to elicit this antibody is conserved in both isoforms, UCP1 and UCP2 of Arabidopsis thaliana.
- Background
-
Background: UCP (uncoupling protein) is an inner membrane mitochondrial protein that can dissipate the proton gradien before it can be used to provide the energy for oxidative phosphorylation. Synonymes: AtUCP, Uncoupling protein 2.
- Product Citations
-
Selected references: Czobor et al. (2019). Comparison of the response of alternative oxidase and uncoupling proteins to bacterial elicitor induced oxidative burst. PLoS One. 2019 Jan 10;14(1):e0210592. doi: 10.1371/journal.pone.0210592.
Takáč et al. (2018). Shot-Gun Proteomic Analysis on Roots of Arabidopsis pldα1 Mutants Suggesting the Involvement of PLDα1 in Mitochondrial Protein Import, Vesicular Trafficking and Glucosinolate Biosynthesis. Int J Mol Sci. 2018 Dec 26;20(1). pii: E82. doi: 10.3390/ijms20010082. (immunolocalization)
Garmash et al. (2017). Expression profiles of genes for mitochondrial respiratory energy-dissipating systems and antioxidant enzymes in wheat leaves during de-etiolation. J Plant Physiol. 2017 Aug;215:110-121. doi: 10.1016/j.jplph.2017.05.023.
Florez-Sarasa et al. (2016). Impaired cyclic electron flow around Photosystem I disturbs high-light respiratory metabolism. Plant Physiol. 2016 Oct 19. pii: pp.01025.2016.
Liu et al. (2015). Silencing of mitochondrial uncoupling protein gene aggravates chilling stress by altering mitochondrial respiration and electron transport in tomato. Acta Physiologiae Plantarum November 2015, 37:223.
Long et al. (2015). Contributions of photosynthetic and non-photosynthetic cell types to leaf respiration in Vicia faba L. and their responses to growth temperature. Plant Cell Environ. 2015 Apr 1. doi: 10.1111/pce.12544.
Grabelnych et al. (2014). Mitochondrial Energy Dissipating Systems (Alternative Oxidase, Uncoupling Proteins, and External NADH Dehydrogenase) Are Involved in Development of Frost Resistance of Winter Wheat Seedlings. ISSN 0006 2979, Biochemistry (Moscow), 2014, Vol. 79, No. 6, pp. 506 519. © Pleiades Publishing, Ltd., 2014.
Barreto et al. (2014). Overexpression of UCP1 in tobacco induces mitochondrial biogenesis and amplifies a broad stress response. BMC Plant Biol. 2014 May 28;14(1):144. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extractsPlant Mitochondria Preparation
In this example of mitochondrial preparation used material are etiolated corn seedlings, however, it can also be applied to prepare crude mitochondria from any plant tissue. The volume has to be adjusted, to match the amount of plant material available. For some plant tissues, it can be useful to include sand when grinding the tissue.
Important remarks before you start:
- Whole procedure should be done at 4ºC
- All centrifugations should be done at 4ºC
- Whole procedure should be done as fast as possible
- Plant material should be homogenized very quickly
- Very careful pipeting is recommended
- After isolation membrane integrity can be checked (should be around 80 %). It can be done using Cytochrome C oxidase kit (Sigma)
Materials:
250 ml grinding media (1.8 g PVPP and 0.34 g L-cysteine)
1 liter beaker and large funnel
2 - large, wetted muslins
8 - 35 ml centrifuge tubes
large pestle and mortar
SS-34 rotor Sorvell centrifuges, or JA-20 for Beckman
75 g corn shootsPrecautions:
Until the time of mitochondrial isolation all plants should be kept in the darkness for min. 8 hours. Plant material should not contain any harder plant parts. Proposed tissue amount 15 grams/60 ml of homogenization buffer. Leaf tissue should be quickly and thoroughly cut followed by 3 minute of homogenization.
It is important to keep all materials cold (4°C) and work swiftly while grinding and spinning.
Procedure:
1. Place ½ of the shoots into the mortar and add grinding media to nearly cover the shoots. Grind until shoots are unrecognizable, add additional grinding medium, and pour pulp into muslin and squeeze. Grind the remaining shoots in a similar way and use the second clean muslin to filter. Divide filtrate evenly among the 8 tubes.
2. Spin at 6500 rpm for 2 min at speed.
3. Transfer supernatant into 8 clean tubes.
4. Spin at 12,750 for 5 min at speed.
5. Discard supernatant. Place the tubes on ice at an angle with pellet-side up.
6. Wash the pellets with 1 ml of Wash Media to remove yellow slime
7. Add 5 ml of Wash Media and resuspend the pellet with a pipetman while leaving behind the small white mass (starch). Pool into two tubes.
8. Spin at 6500 rpm for 2 min at speed.
9. Pipet supernatant into clean tubes while leaving behind slimy film
10. Underlay the supernatant with 8 ml of Sucrose cushion.
11. Spin at 9250 rpm for 20 min at speed.
12. Aspirate supernatant.
13. Resuspend each pellet in a suspension medium of choice, this is your crude mitochondrial prearation.Solutions Required
GRINDING MEDIUM: 350 mM Mannitol; 30 mM Mops, 1 mM EDTA, pH 7.6
1 Liter
Mannitol 63.8 g
Mops 6.25 g
1 mM EDTA 2 ml of 0.5 M stock
WASH MEDIUM: 300 mM Mannitol, 20 mM Mops, 1 mM EDTA, pH 7.2250 mls
Mannitol 13.7 g
Mops 1.05 g
1 mM EDTA 0.5 ml of 0.5 M stock
SUCROSE CUSHION: 0.6 M Sucrose100 mls
Sucrose 20.5 g
SUSPENSION MEDIUM: 250 mM Sucrose, 30 mM Mops, pH 7.2100 ml
Sucrose 8.56 g
Mops 0.628 gCourtesy Dr. Thomas Elthon
- Reviews:
-
This product doesn't have any reviews.