2
Anti-SUN1,2 (nuclear envelope protein) (Arabidopsis thaliana)
AS15 2856 | Clonality: Polyclonal | Host: Rabbit | Reactivity: Arabidopsis thaliana
- Product Info
-
Immunogen: KLH-conjugated synthetic peptide derived from SUN1,2 sequences of Arabidopsis thaliana SUN1 UniProt: Q9FF75, TAIR: AT5G04990, SUN2, UniProt: Q9SG79, TAIR: AT3G10730
Host: Rabbit Clonality: Polyclonal Purity: Immunogen affinity purified serum in PBS pH 7.4. Format: Lyophilized Quantity: 50 µg Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Western blot (WB), Immunocytochemistry (ICC) Recommended dilution: 1 : 1000 (WB), 1 : 200 (ICC) Expected | apparent MW: 51.5 kDa (SUN1); 49.9 kDa (SUN2) - Reactivity
-
Confirmed reactivity: Arabidopsis thaliana Predicted reactivity: Arabidopsis thaliana - Application Examples
-
Application example 
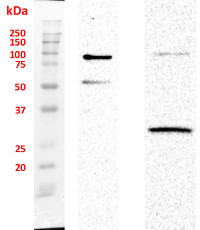
Chloroplast fraction was obtained using Chloroplast Isolation Kit (CPISO-1KT, Sigma-Aldrich). 1. Supernatant proteins after DNA digestion, 2. Unquantified, 10 µl of pellet after DNA digestion. Signal of SMT1 (Sterol methyltransferase 1, marker antibody of integral ER membrane) was not detected. Nuclei fraction was isolated from 1 g fresh weight of 3-4 old day cell suspension culture of Arabidopsis thaliana Ler according to protocol published by Xu and Copeland (2012). Nuclei fraction was stored in NSB buffer (with added ROCHE and PMSF inhibitors) in 80 °C. Residue of nuclei was centrifuged and pellet was re-suspended in 100 µl of modified RIPA buffer with added protease inhibitors and incubated 1h on ice. Then 20 µl of DNase I was added into each sample and all samples were incubated on ice for 45 min. After that 45 min another 20 µl of DNase I was added and incubated under the same conditions. Samples were twice centrifuged 1) 15 000 × g for 15 min and 2) 30 000 × g for 30 min. Pellets from the first step of centrifugation was stored. Proteins in second supernatant were precipitated with ice-cold acetone over night and twice washed. Protein pellet was dissolved in buffer contained 6 M Urea, 2 M Thiourea and 25 mM Tris, pH = 7,5. Proteins were quantified by using Bradford method. Totally 3 µg of protein was loaded on gel. Pellet after DNase treatment was dissolved in 20 µl of LSB buffer. Samples were boiled on 95°C for 10 min before loading on 4 / 12% polyacrylamide gel. Electrophoretic conditions: 90 V during whole separation (2 h); Transfer conditions: 90 mA, 30 V over night, nitrocellulose membrane; Blocking: 1 h, 5% low fat milk; Primary antibody incubation: 1 h at a dilution of 1: 1000; Washes: 3 × 5 min.; Secondary antibody: 1 h at a dilution of 1: 5000; washes: 3 × 10 min. ELC substrate was loaded on membrane and signals were detected with ChemiDoc system (Bio-Rad).
Courtesy of Dr. Martin Kubeš, Centre of the Region Haná for Biotechnological and Agricultural Research, Department of Chemical Biology and Genetics, Olomouc, Czech Republic and Vladimír Skalický, Laboratory of Growth Regulators, Faculty of Science of Palacký University and Institute of Experimental Botany ASCR, Olomouc, Czech Republic
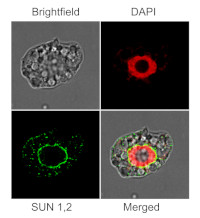
Immunofluorescent localization of SUN1,2 on suspension culture of Arabidopsis thaliana, using anti-SUN1,2 antibodies (AS15 2856) and anti-rabbit IgG DyLight®488 conjugated secondary antibodies (AS10 1165). DAPI staining of nuclei is pseudocolored red.
Material: Suspension cultures of Arabidopsis thaliana, ecotype Landsberg erecta cv.MM1
Fixation: Packed cell volume to fixer ratio: 250 µl : 5ml
Fixer composition and buffer: 4% (w/v) paraformaldehyde (freshly prepared as 8% stock and 0.2 µm filtered) in Phosphate Buffered Saline (PBS), pH 7.4 (2x stock, 0.2 µm filtered)
Container and method: in 6 cm Petri dish, gentle shaking at room temperature (RT)
Duration: 30 minutes. Cells were not shaken during the first 5 mins of fixation to allowed to partially recover from osmotic shock induced by formaldehyde.
Hydrophilization: no
Cell wall digestion: Yes
Packed cell volume to enzyme ratio: 100 µl : 2ml Enzyme composition: 1% (A) 1.2% (R) Cellulase (chromatically purified, powder, Worthington) 1% Pectinase (protease free, liquid, Sigma) Buffer: 0.5% (w/v) MES buffer, pH 5.6
Container and method: in 2 ml microfuge tube by rolling at room temperature (RT)
Duration: 30 minutes
Membrane permeabilization: Triton-X100 (0.5%), 10 min/RT
Antigen retrieval: no
Blocking buffer: Fish gelatin (5% v/v)
Washing buffer: PBS
Primary antibody dilution and incubation time: 1:400, ON/4ºC
Secondary antibody dilution and incubation time and supplier: anti-rabbit IgG DyLight®488 conjugated secondary antibodies (AS10 1165), 1:600, 1hn/RT
Co-staining of the nucleus (DAPI): Yes
Nucleus staining: 100 ng/ml DAPICourtesy of Dr. Ferhan Ayaydin, Hungarian Centre of Excellence for Molecular Medicine (HCEMM), Szeged, Hungary.
- Additional Information
-
Additional information (application): For other species use product AS15 2857
This antibody is not suitable for immunolocalization. - Background
-
Background: SUN1,2 (nuclear envelope protein) is a member of the Sad1/UNC-84 (SUN)-domain proteins.SUN domain proteins are part of the cytoskeletal-nucleoskeletal bridging complexes. These proteins are localized to the nuclear envelope and are present as homomers and heteromers in vivo. Involved in maintaining the elongated nuclear shape of epidermal cells. - Product Citations
-
Selected references: Wang et al. (2019) OPENER Is a Nuclear Envelope and Mitochondria Localized Protein Required for Cell Cycle Progression in Arabidopsis. Plant Cell. 2019 Jul;31(7):1446-1465. doi: 10.1105/tpc.19.00033 - Protocols
-
Agrisera Western Blot protocol and video tutorials
Preparation of cytosolic and nuclear protein fractions
1. Prepare protoplasts from 50 ml Arabidopsis thaliana cell culture according to the protocol of PEG transfection.
2. Resuspend protoplasts in 10 ml GH buffer and keep the solution on ice for 10 min.
GH buffer: 100mM glycine
0.1% Hexylene glycol
0.37M (4.7% w/v) saccharose
0.3mM Spermine
1.0mM Spermidine
pH 8.3 with Ca(OH)2
3. To release nuclei add Triton X100 to a final concentration of 0.1%. Pipetting gently up and down several
times with a plastic pipette might be necessary to lyse cells.
4. After 5min sediment nuclei by centrifugation at 1000 xg for 15 min at 4°C. Save supernatant as the
cytoplasmic fraction. Wash the pelleted nuclei two times with GHT (GH+0.1% TX100) then finally
resuspended in a suitable volume of extraction buffer + protease inhibitors.Courtesy Dr. Laszlo Bako, Umeå Plant Science Centre, Sweden
- Reviews:
-
This product doesn't have any reviews.



