1
Anti-PsbS | 22 kDa Lhc-like PSII protein (rabbit antibody)
AS09 533 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. thaliana, B. corticulans, B. distachyon, C. cantabricus (Wilk.) Rchb. F, F.a bidentis, H. pilosella L., H. vulgare, L. hispanica, M. esculenta, N. tabacum, O. sativa, P. abies, P. glauca, P. strobus, R.communis, S.oleracea, S. muralis (Hedw.) Raab, T. aestivum, Z.mays
- Product Info
-
Immunogen: KLH-conjugated synthetic peptide located in solubilized part of the protein, derived from available di- and monocot PsbS sequences, including Arabidopsis thaliana UniProt:Q9XF91, TAIR:At1g44575
Host: Rabbit Clonality: Polyclonal Purity: Serum Format: Lyophilized Quantity: 50 µl Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Western blot (WB) Recommended dilution: 1 : 2000 - 1: 10 000 (WB) Expected | apparent MW: 28 | 22 kDa for Arabidopsis thaliana
- Reactivity
-
Confirmed reactivity: Arabidopsis thaliana, Brachypodium distachyon, Bryopsis corticulans, Cytisus cantabricus (Wilk.) Rchb. F, Deschampsia antartica, Flaveria bidentis, Hieracium pilosella L., Hordeum vulgare, Lasallia hispanica, Manihot esculenta, Nicotiana benthamiana, Nicotiana tabacum, Oryza sativa, Picea abies, Picea glauca, Pinus strobus, Ricinus communis, Setaria viridis, Spinacia oleracea, Syntrichia muralis (Hedw.) Raab, Tillandsia flabellate, Triticum aestivum, Zea mays Predicted reactivity: Chlamydomonas reinhardtii, Cucumis sativus, Gossypium hirsutum, Medicago truncatula, Mesotaenium braunii, Physcomitrium patens, Picea sitchensis, Pinus radiata, Pinus taeda, Populus balsamifera, Solanum lycopersicum, Spirogyra sp., Tarenaya hassleriana, Zosteria marina, Vitis vinifera
Species of your interest not listed? Contact usNot reactive in: Lobosphaera incisa, Ostreococcus tauri - Application Examples
-

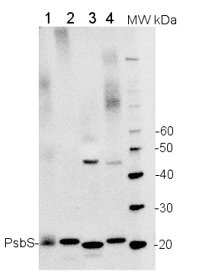
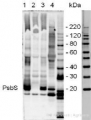
5 µg of total extract from (1) Arabidopsis thaliana leaf, (2) Spinacia oleracea (3) Hordeum vulgare (4) Zea mays extracted with PEB (AS08 300) were separated on 4-12% NuPage (Invitrogen) LDS-PAGE and blotted 1h to PVDF. Blots were blocked immediately following transfer in 2% ECL Advance blocking reagent (GE Healthcare) in 20 mM Tris, 137 mM sodium chloride pH 7.6 with 0.1% (v/v) Tween-20 (TBS-T) for 1h at room temperature with agitation. Blots were incubated in the primary antibody at a dilution of 1: 10 000 for 1h at room temperature with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at room temperature with agitation. Blots were incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated) diluted to 1:50 000 in 2% ECL Advance blocking solution for 1h at room temperature with agitation. The blots were washed as above and developed for 5 min with ECL Advance detection reagent according to the manufacturers instructions. Images of the blots were obtained using a CCD imager (FluorSMax, Bio-Rad) and Quantity One software (Bio-Rad). Exposure time was 30 seconds.

50 mg of leaf tissue from 3-4 week old Arabidopsis thaliana (columbia) wilde type (1,2) or NPQ4 (3,4) was ground to fine powder in liquid nitrogen with a small plastic grinder and further homogenized in 200 ul of 1x Sample Loading Buffer (12 % glycerol, 60 mM Tris-HCl pH 6.8, 2.2 % SDS, 0.04 % bromophenol blue, 1.2 % beta-mercaptoethanol). Samples were heated at 95C for 1.5 minute and spun 30 seconds at 12k rpm. 10 ul of total protein extract was loaded onto BoltTM 4-12 % Bis-TrisPlusGels (Invitrogen) and run for 40 min. at 165 V and transferred to nitrocellulose in mini Bolt module for 1 h at 10V. Blot was blocked in 5 % nonfat dry milk in TBST (NaCl 137 mM, KCl 2.7 mM, Tri base 19 mM) and incubated with primary antibodies at 1: 1000 dilution for 1 hour at RT. After 4x5 minute wash in TBST, secondary antibody incubation (goat anti-rabbit IgG HRP conjugated, Agrisera AS09 602) at a dilution of 1: 10 000 for 1 h was follwed by 4x5 min. washes. Blots were well drained and incubaed briefly with PierceSuperSignalWest Pico Chemiluminescent Sustraste before exposure to ImageQuant ccd camera
Courtesy of Dr. Laura Roy, University of Amsterdam, The Netherlands
10 µL of total protein extracted freshly from Arabidopsis thaliana (A), Brachypodium distachyon (B) and Oryza sativa (R) with 1X Laemmli Buffer (63 mM Tris-HCl pH 6.8, 10% (w/v) glycerol, 2% SDS and Bromophenol Blue q.b./1 pick) diluted to 1:2 and denatured with 25 mM DTT at 95ºC for 4 min, were resolved on a 10 % SDS-PAGE and blotted 1h to PVDF membrane (pore size of 0.45 μm, GE Healthcare), using wet transfer. Blot was blocked with Blocking solution containing 5% milk in TBS-T, for 1h/RT with agitation. Membrane was incubated with the primary antibody at a dilution of 1:2000 in Blocking solution containing 5% milk in TBS-T, for 1h/RT with agitation in TBS-T. The antibody solution was removed, and the membrane was washed 3 times for 10 min in TBS-T at RT with agitation. The membrane was then incubated in the secondary antibody (anti-rabbit IgG, horse radish peroxidase conjugated) diluted to 1:20 000 in Blocking solution containing 5% milk in TBS-T, for 1h/RT with agitation. After incubation with the secondary antibody, membrane was washed 6 times for 5 min in TBS-T at RT with agitation and developed for 1 min with Agrisera ECLSuperBright. Exposure time was 10 seconds.
Courtesy of Dr. Nelson Saibo,Plant Gene Regulation Lab (GPlantS Unit), ITQB NOVA, Portugal
Application examples: 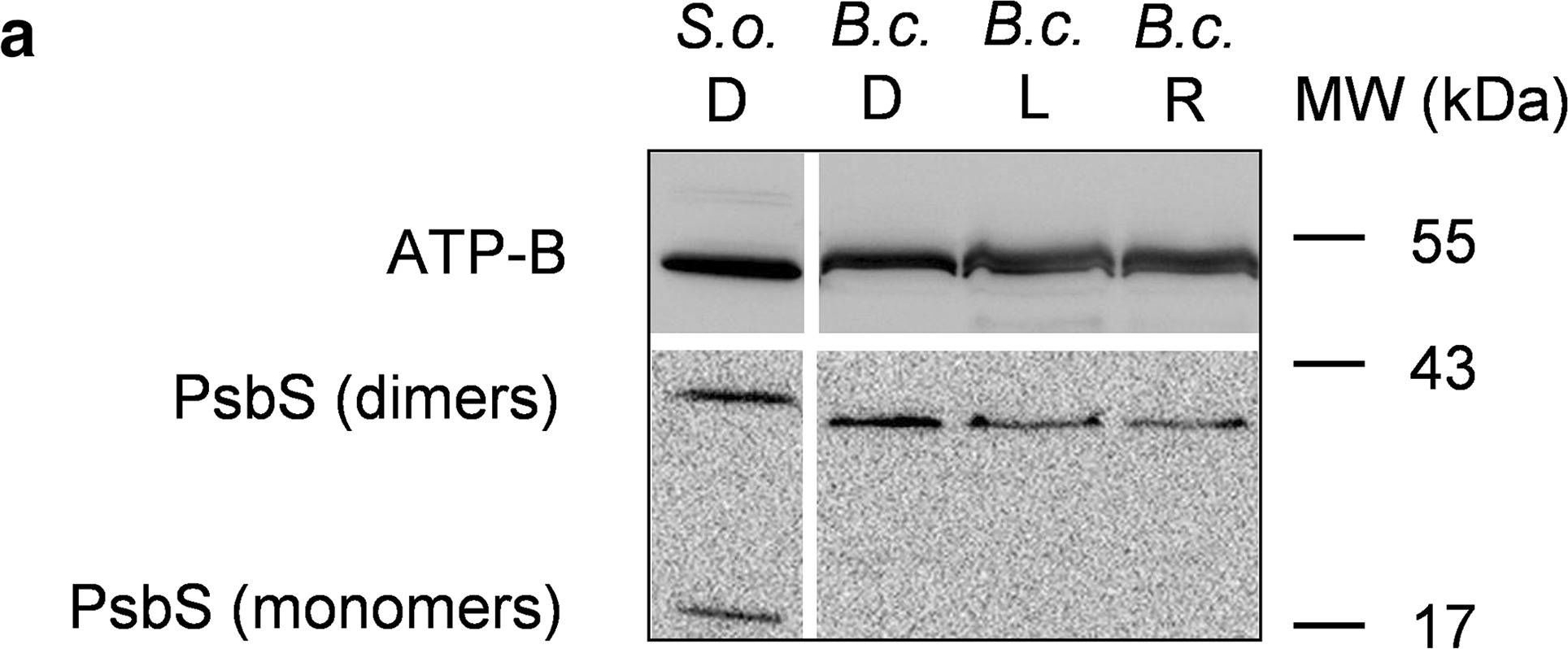
Reactant: Algae
Application: Western Blotting
Pudmed ID: 29460179
Journal: Planta
Figure Number: 7A
Published Date: 2018-06-01
First Author: Giovagnetti, V., Han, G., et al.
Impact Factor: 3.95
Open PublicationWestern blot analysis of PsbS and LHCSR proteins from dark-adapted (D, 30 min), light-treated (L, 2500 µmol photons m?2 s?1, 30 min) and post-dark-recovery chloroplasts (R, 1 h) isolated from B. corticulans. Samples were analysed by immunoblotting with an anti-PsbS (a), anti-LHCSR3 (b) and anti-LHCSR1 antibodies (c). Control samples are dark-adapted S. oleracea (a) and C. reinhardtii chloroplasts (b, c). Note that B. corticulans and S. oleracea samples have been probed against anti-PsbS antibody in the same membrane. The separation between lanes is only due to the presence of different samples between those presented of S. oleracea and B. corticulans (a). The ? subunit of ATP synthase (ATP-B) was used as loading control. 10 µg of Chl was loaded in each lane
Reactant: Nicotiana tabacum (Common tobacco)
Application: Western Blotting
Pudmed ID: 29511193
Journal: Nat Commun
Figure Number: 1C
Published Date: 2018-03-06
First Author: G?owacka, K., Kromdijk, J., et al.
Impact Factor: 13.783
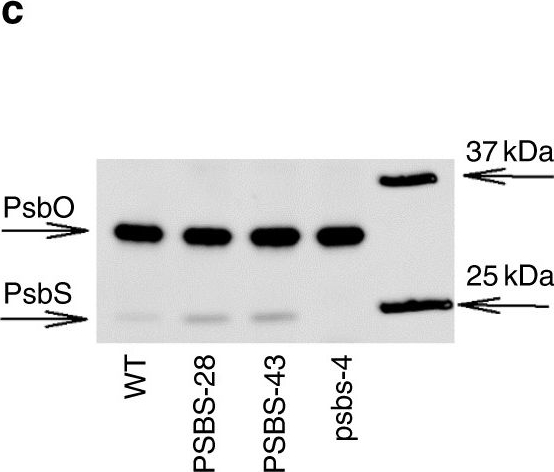
Open PublicationPhotosynthesis and water-use efficiency in Nicotiana tabacum plants with modified PsbS levels. aPsbS mRNA levels normalized to actin and tubulin sampled from fully expanded leaves of psbs-4, PSBS-28, PSBS-43 and wild-type (WT) N. tabacum plants. b PsbS protein levels normalized to the large subunit of oxygen-evolving complex of photosystem II (PsbO), determined from densitometry on immunoblots. c Representative immunoblot for PsbS and PsbO. d Net CO2 fixation rate (An), e NPQ, f quinone A (QA) redox state, and g stomatal conductance (gs) as a function of incident light intensity in fully expanded leaves. h Linear correlation between QA redox state and gs. Broken lines indicate measurements at highest light intensity. i Linear correlation between PsbS protein levels and intrinsic water-use efficiency (An/gs) at light intensity above 600??mol?m?2?s?1. Asterisks/lines show significant differences from WT (black for silencing, red for overexpressing lines; Dunnett’s two-way test; ??=?0.05). Error bars indicate s.e.m. (n?=?from 6 to 10 biological replicates)
Reactant: Solanum lycopersicum (Tomato)
Application: Western Blotting
Pudmed ID: 34439953
Journal: Biology (Basel)
Figure Number: 9A
Published Date: 2021-07-28
First Author: Trojak, M. & Skowron, E.
Impact Factor: None
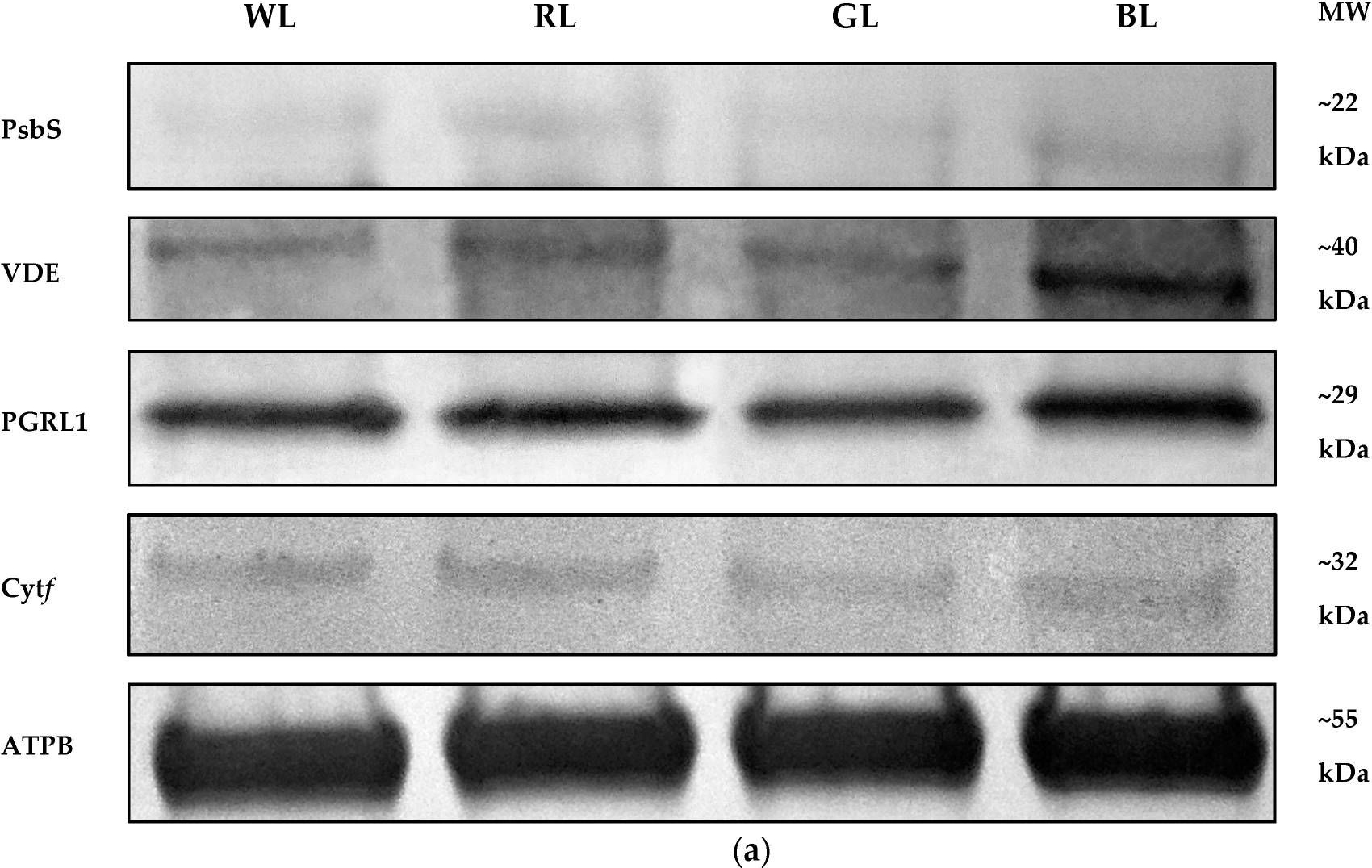
Open PublicationWestern Blot analyses (a) and densitometric analyses of PsbS (b), VDE (c), PGRL1 (d), and cytf (e) proteins in leaves of tomato plants (Solanum lycopersicum L. cv. Malinowy Ozarowski) grown under different light conditions (see Material and Methods for details). The bands were normalized to the appropriate ? subunit of ATP synthase (ATPB) band (loading control, Figure S4) (a). The bar diagrams (b–e) represent pixel volumes (densitometric analyses) of proteins in samples. Each value represents the mean ± SD (n = 3) considering the control sample (WL) value as 1 (100%). Different letters indicate significant differences between treatments (p = 0.05) with a Tukey’s HSD test. MW—molecular weight.
- Additional Information
-
Additional information (application): This product can be sold containing proclin if requested - Background
-
Background: The 22 kDa PsbS protein of photosystem II functions in the regulation of photosynthetic light harvesting. Along with a low thylakoid lumen pH and the presence of de-epoxidized xanthophylls, PsbS is necessary for photoprotective thermal dissipation of excess absorbed light energy in plants, measured as non-photochemical quenching of chlorophyll fluorescence. Synonymes: NPQ4 (NONPHOTOCHEMICAL QUENCHING).
- Product Citations
-
Selected references: Chen et al. (2024). Distinct features of PsbS essential for mediating plant photoprotection. Plant Commun. 2024 Oct 28:101179. doi: 10.1016/j.xplc.2024.101179.
Frangedakis et al. (2024). MYB-related transcription factors control chloroplast biogenesis. Cell: DOI:https://doi.org/10.1016/j.cell.2024.06.039.
Ermakova et al. (2024). Chloroplast NADH dehydrogenase-like complex-mediated cyclic electron flow is the main electron transport route in C4 bundle sheath cells. New Phytol. 2024 Jul 22.doi: 10.1111/nph.19982.
Turc et al. (2024). Non-photochemical quenching upregulation improves water use efficiency and reduces whole plant level water consumption under drought. J Exp Bot. 2024 Mar 12:erae113. doi: 10.1093/jxb/erae113.
Völkner et al. (2024). Evidence for partial functional overlap of KEA and MSL transport proteins in the chloroplast inner envelope of Arabidopsis thaliana. FEBS Lett. 2024 Aug;598(15):1877-1887. doi: 10.1002/1873-3468.14887.
Hu C, Mascoli V, Elias E, Croce R. The photosynthetic apparatus of the CAM plant Tillandsia flabellate and its response to water deficit. J Plant Physiol. 2023 Mar;282:153945. doi: 10.1016/j.jplph.2023.153945
Jiang et al. (2020). Plastid chaperone HSP90C guides precursor proteins to the SEC translocase for thylakoid transport. J Exp Bot. 2020 Aug 27;eraa399.doi: 10.1093/jxb/eraa399.
Barbato et al. (2020). Higher Order Photoprotection Mutants Reveal the Importance of ?pH-dependent Photosynthesis-Control in Preventing Light Induced Damage to Both Photosystem II and Photosystem I. Sci Rep . 2020 Apr 21;10(1):6770. doi: 10.1038/s41598-020-62717-1.
Nikkanen et al. (2018). Multilevel regulation of non-photochemical quenching and state transitions by chloroplast NADPH-dependent thioredoxin reductase. Physiol Plant. 2018 Dec 22. doi: 10.1111/ppl.12914.
Chen et al. (2018). Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol Plant. 2018 Apr 6. doi: 10.1111/ppl.12737.
Glowacka et al. (2018). Photosystem II Subunit S overexpression increases the efficiency of water use in a field-grown crop. Nat Commun. 2018 Mar 6;9(1):868. doi: 10.1038/s41467-018-03231-x.
Giovagnetti et al. (2018). A siphonous morphology affects light-harvesting modulation in the intertidal green macroalga Bryopsis corticulans (Ulvophyceae). Planta. 2018 Feb 19. doi: 10.1007/s00425-018-2854-5.
Chen et al. (2017). Comparison of Photosynthetic Characteristics and Antioxidant Systems in Different Wheat Strains. J Plant Growth Regul.c
Merry et al. (2017). A comparison of pine and spruce in recovery from winter stress; changes in recovery kinetics, and the abundance and phosphorylation status of photosynthetic proteins during winter. Tree Physiol. 2017 Sep 1;37(9):1239-1250. doi: 10.1093/treephys/tpx065.
Krishnan et al. (2017). Large-scale in vitro production, refolding and dimerization of PsbS in different microenvironments. Sci Rep. 2017; 7: 15200. Published online 2017 Nov 9.
Míguez et al. (2017). Diversity of winter photoinhibitory responses: A case study in co-occurring lichens, mosses, herbs and woody plants from subalpine environments. Physiol Plant. 2017 Feb 14. doi: 10.1111/ppl.12551.
Yoshida et al. (2016). Hisabori T1.Two distinct redox cascades cooperatively regulate chloroplast functions and sustain plant viability. Proc Natl Acad Sci U S A. 2016 Jul 5;113(27):E3967-76. doi: 10.1073/pnas.1604101113. Epub 2016 Jun 22.
Poudyal et al. (2016). Production of superoxide from photosystem II-light harvesting complex II supercomplex in STN8 kinase knock-out rice mutants under photoinhibitory illumination. J Photochem Photobiol B. 2016 Sep;162:240-7. doi: 10.1016/j.jphotobiol.2016.06.050.
Ishikawa et al. (2016). NDH-Mediated Cyclic Electron Flow Around Photosystem I is Crucial for C4 Photosynthesis. Plant Cell Physiol. 2016 Aug 6. pii: pcw127. [Epub ahead of print]
Pavlovic et al. (2016). Light-induced gradual activation of photosystem II in dark-grown Norway spruce seedlings. Biochim Biophys Acta. 2016 Feb 18. pii: S0005-2728(16)30028-7. doi: 10.1016/j.bbabio.2016.02.009.
Karlsson et al. (2015). The Arabidopsis thylakoid transporter PHT4;1 influences phosphate availability for ATP synthesis and plant growth. Plant J. 2015 Aug 8. doi: 10.1111/tpj.12962.
Dahal et al. (2015). Improved photosynthetic performance during severe drought in Nicotiana tabacum overexpressing a nonenergy conserving respiratory electron sink. New Phytol. 2015 May 29. doi: 10.1111/nph.13479.
Belgio et al. (2015). Light harvesting superstructures of green plant chloroplasts lacking photosystems. Plant Cell Environ. 2015 Mar 4. doi: 10.1111/pce.12528.
Lintala et al. (2013). Arabidopsis tic62 trol mutant lacking thylakoid bound ferredoxin-NADP+ oxidoreductase shows distinct metabolic phenotype. Mol Plant Sep 16.
Zienkiewicz et al. (2013).Light intensity and quality stimulated Deg1-dependent cleavage of PSII components in the chloroplasts of maize. Plan Physiol Biochem. March 16.
Albus et al. (2012). LCAA, a novel factor required for Mg protoporphyrin monomethylester cyclase accumulation and feedback-control of aminolevulinic acid biosynthesis in tobacco. Plant Physiol. Oct 19. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Oxygenic photosynthesis poster by prof. Govindjee and Dr. Shevela
Z-scheme of photosynthetic electron transport by prof. Govindjee and Dr. Björn and Dr. Shevela - Reviews:
-
Tim | 2025-04-14We tested a panel of antibodies targeting various photosynthetic proteins, including both light-harvesting complex proteins (e.g., Lhcb1 or Lhcb2) and core complex components (e.g., PsbC). Across all experiments, the antibodies demonstrated strong specificity and reliable performance. The resulting immunoblot signals were clear, well-defined, and reproducible, with minimal background or non-specific binding.amil Trzebuniak | 2020-03-06I have used this antibody to distinguish between Wild Type Arabidopsis thaliana and PsbS-lacking mutants. For my Western Blott I have used the antibody with the recommended dilution (1:2000) while the total protein load was 20 ug per well. The obtained results were really nice there was a clear band in the T plants and no signal was obtained from the mutant plants.
Kamil,
Jagiellonian University
Soo Yeon Ko | 2020-02-27This antibody worked in A. thaliana and Oryza Sativa in 1:5000 dilutions. We could see one specific band clearlyKatarzyna Hura | 2013-10-30We have used this antibody for western blot detection in wheat and barley (drought stress and cold acclimation). I have been using recommended dilution 1:40 000.Maksymilian Zienkiewicz | 2012-01-16This antibody worked efficiently both in A. thaliana and Zea mays plants in suggested working dilutions. There was visible one specific band.
Accessories
AS03 032 | Clonality: Polyclonal | Host: Hen | Reactivity: A. thaliana, A. sativa, B. napus, H. vulgare, O. sativa, P. sylvestris, P. tremula, S. oleracea


