1

Anti-PsbB | CP47 protein of PSII
AS04 038 | Clonality: Polyclonal | Host: Rabbit | Reactivity: [global antibody] for higher plants, Physcomitrella patens, algae, cyanobacteria, diatoms
Benefits of using this antibody
- Product Info
-
Immunogen: KLH-conjugated synthetic peptide derived from available plant, algal and cyanobacterial PsbB sequences including Arabidopsis thaliana AtCg00680, Hordeum vulgare P10900, Oryza sativa P0C364, Synechocystis PCC 6803 P05429
Host: Rabbit Clonality: Polyclonal Purity: Serum Format: Lyophilized Quantity: 50 µl Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Clear-native PAGE (CN-PAGE), Western blot (WB) Recommended dilution: 1: 10 000 (CN-PAGE), 1 : 2000 (WB) Expected | apparent MW: 56 kDa
- Reactivity
-
Confirmed reactivity: Anabaena 7120, Arabidopsis thaliana, Chlamydomonas reinhardtii, Dionaea muscipula, Echinochloa crus-galli, Hordeum vulgare, Malus prunifolia, Mesostigma viride, Nicotiana benthamiana, Opephora guenter-grassii (diatom), Oryza sativa, Panicum miliaceum, Phaseolus vulgaris, Physcomitrium patens, Pisum sativum, Skeletonema costatum (diatom), Synechococcus PCC7942, 6803, , Seminavis robusta (diatom), Zea mays Predicted reactivity: Abies concolor, Brachypodum distachyon, Brassica napus, Cannabis sativa, Cyanobacteria, Cucumis sativus, Ephedra sp., Glycine max, Lotus japonicus, Manihot esculenta, Nanochloropsis sp., Nicotiana tabacum, Panax ginseng, Populus trichocarpa,
Species of your interest not listed? Contact usNot reactive in: No confirmed exceptions from predicted reactivity are currently known - Application Examples
-
Application example
.jpg)
2 µg of total protein from Arabidopsis thaliana leaf (1), Horderum vulgare (2), Chlamydomonas reinhardtii total cell (3) Synechococcus sp. 7942 total cell (4), Anabaena sp. total cell (5), were extracted with PEB (AS08 300) and separated on 4-12% NuPage (Invitrogen) LDS-PAGE and blotted 1h to PVDF. Blots were blocked immediately following transfer in 2% blocking reagent in 20 mM Tris, 137 mM sodium chloride pH 7.6 with 0.1% (v/v) Tween-20 (TBS-T) for 1h at room temperature with agitation. Blots were incubated in the primary antibody at a dilution of 1: 50 000 for 1h at room temperature with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at room temperature with agitation. Blots were incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, recommended secondary antibody AS09 602) diluted to 1:50 000 in 2% blocking solution for 1h at room temperature with agitation. The blots were washed as above and developed for 5 min with chemiluminescence detection reagent according the manufacturers instructions. Images of the blots were obtained using a CCD imager (FluorSMax, Bio-Rad) and Quantity One software (Bio-Rad).
2.0 µg of chlorophyll from Pisum sativum chloroplasts and from Zea mays, Echinochloa crus-galli, Panicum miliaceum mesophyll and bundle sheath chloroplasts extracted with 0.4 M sorbitol, 50 mM Hepes NaOH, pH 7.8, 10 mM NaCl, 5 mM MgCl2 and 2 mM EDTA. Samples were denatured with Laemmli buffer at 75°C for 5 min and were separated on 12% SDS-PAGE and blotted 30 min to PVDF using wet transfer. Blot was blocked with 5% milk in TBS for 2h at room temperature (RT) with agitation. Blot was incubated in the primary antibody AS04 038 at a dilution of 1: 2000 overnight at 4°C with agitation in 1% milk in TBS-T. The antibody solution was decanted and the blot was washed 4 times for 5 min in TBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, from Agrisera, AS09 602) diluted to 1:25 000 in 1% milk in TBS-T for 1h at RT with agitation. The blot was washed 5 times for 5 min in TBS-T and 2 times for 5 min in TBS, and developed for 1 min with 1.25 mM luminol, 0.198 mM coumaric acid and 0.009% H2O2 in 0.1 M Tris- HCl, pH 8.5. Exposure time in ChemiDoc System was 122 seconds.
Courtesy Dr. Wioleta Wasilewska-Dębowska, Warsaw University, PolandApplication examples: 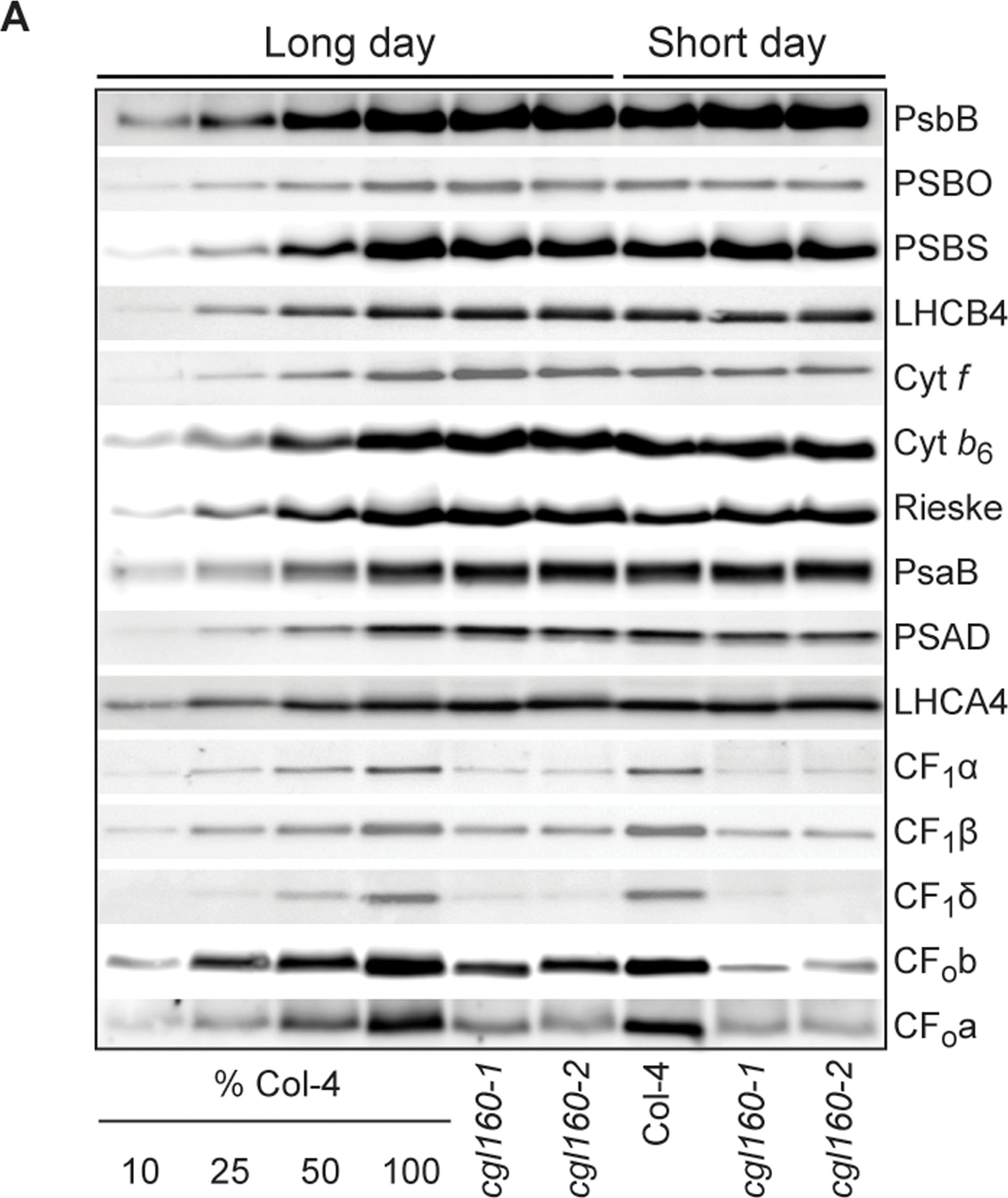
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 25835989
Journal: PLoS One
Figure Number: 7A
Published Date: 2015-04-04
First Author: Fristedt, R., Martins, N. F., et al.
Impact Factor: 2.942
Open PublicationAltered protein accumulation and stability of the chloroplast ATP synthase in the cgl160 mutant visualized by immunoblotting.A. Immunoblots with antibodies against essential subunits of the photosynthetic protein complexes of wild-type (Col-4) Arabidopsis and the two cgl160 T-DNA insertion lines grown under long-day and short-day conditions. Isolated thylakoid membranes were used, and equal amounts of chlorophyll were loaded onto the SDS-PAGE gel. For approximate quantification, wild-type samples from long-day plants were diluted to 10%, 25% and 50%, respectively. Accumulation of PSII was probed with antibodies against PsbB and PSBO. Additionally, the PSBS protein involved in NPQ and the minor PSII antenna protein LHCB4 were probed. Accumulation of the cytochrome b6f complex was probed with antibodies against the essential subunits PetA (cytochrome f), PetB (cytochrome b6), and PETC (Rieske protein). Accumulation of PSI was probed with antibodies against the reaction center subunit PsaB and the stromal ridge subunit PsaD. ATP synthase accumulation was probed with antibodies against the CF1 subunits AtpA (CF1?), AtpB (CF1?) and AtpD (CF1?) and antibodies against the CF0 subunits AtpF (CF0b) and AtpI (CF0a). B. Loading difference estimation for immunoblotting CF1 between wild type and cgl160-1. To obtain similar immunoblotting signal three times more (15 ?g protein) was needed for cgl160-1 compared to wild type (5 ?g protein). C. Maintenance of CF1 was measured by incubating leaves from wild type and cgl160-1 in solution containing the plastid protein synthesis inhibitor chloramphenicol for the indicated time points. Protein extract was isolated and separated by SDS-PAGE, immunoblotted and probed with specific antibodies against CF1 and LHCB2.1. Three times more protein was loaded from the mutant to obtain equal level of CF1 immunoblotting signal, as specified in B.
Reactant: Nicotiana tabacum (Common tobacco)
Application: Western Blotting
Pudmed ID: 28180288
Journal: J Exp Bot
Figure Number: 5A
Published Date: 2017-02-01
First Author: Schöttler, M. A., Thiele, W., et al.
Impact Factor: 6.088
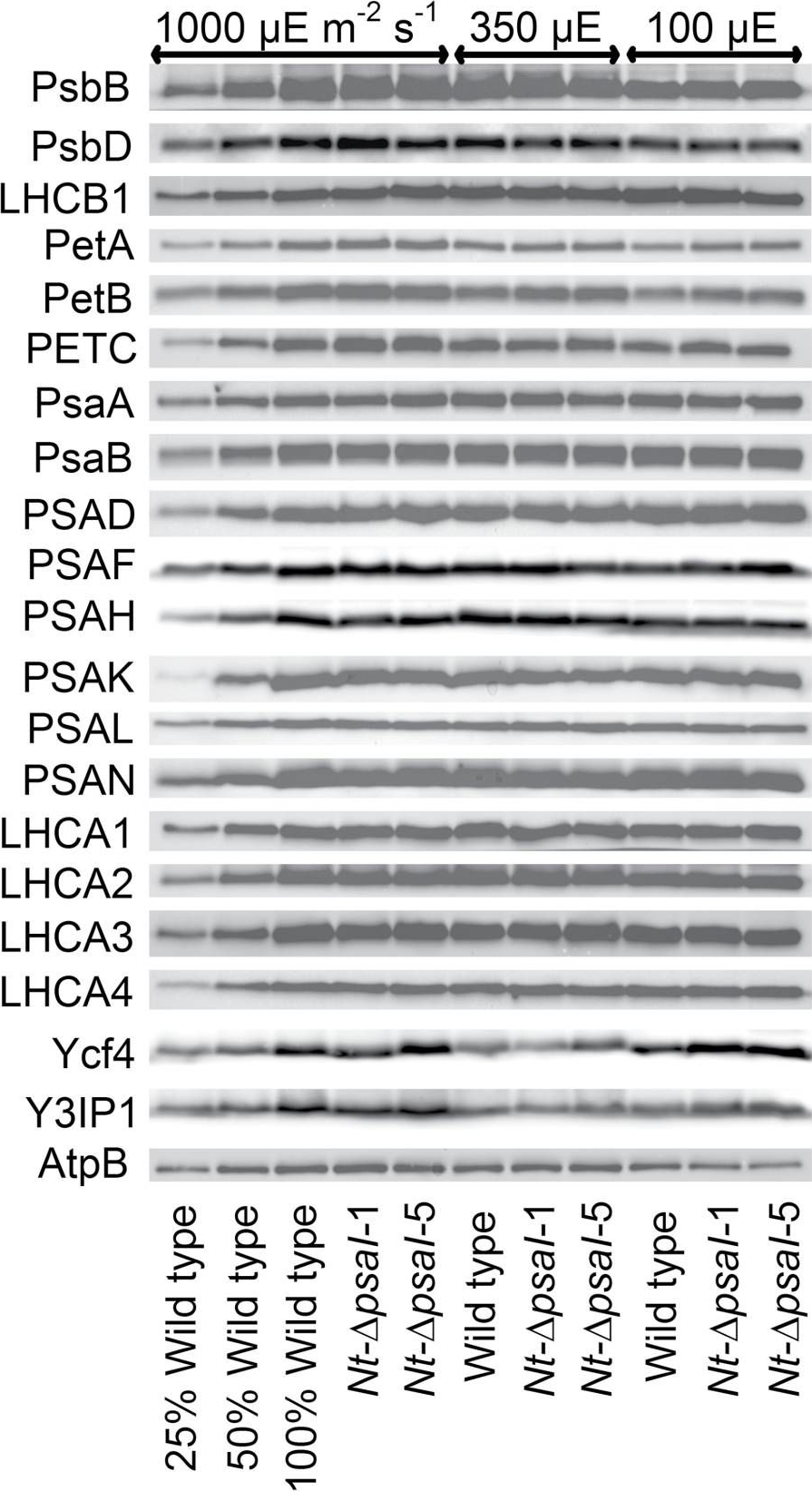
Open PublicationImmunoblot analysis of photosynthetic complex accumulation in wild-type tobacco and the two ?psaI lines grown under low, intermediate, and high-light conditions. Because the accumulation of most tested proteins was highest under high-light conditions, lanes one to three contain samples diluted to 25%, 50%, and a 100% sample of wild-type tobacco grown under high-light conditions, to allow for semi-quantitative determination of changes in protein abundance. Lanes four and five contain the two transplastomic lines grown at 1000 µE m?2 s?1. Lanes six to eight contain wild-type tobacco and the mutants grown at intermediate light intensities, and lanes nine to eleven contain samples grown at low light intensities. For PSII, the accumulation of the essential subunits PsbB (CP43) and PsbD (D2) and the LHCB1 antenna protein were determined, while for the cytochrome b6f complex, the accumulation of the essential redox-active subunits PetA (cytochrome f), PetB (cytochrome b6), and PETC (Rieske FeS protein) was tested. AtpB was probed as an essential subunit of the chloroplast ATP. For PSI, in addition to the three essential plastome-encoded subunits PsaA, PsaB, and PsaC, the accumulation of the nuclear-encoded subunits PSAD, PSAH, PSAK, PSAL, and PSAN and of the four LHCI proteins (LHCA1, LHCA2, LHCA3, LHCA4) was determined. Finally, we examined the accumulation of Ycf4, the chloroplast-encoded PSI-biogenesis factor encoded in the same operon as PsaI, and the nuclear-encoded assembly factor Y3IP1.
- Additional Information
-
Additional information: This antibody can be used as a loading control for studies of PSIi or photosynthetic acclimation in diatoms Blommaert et al. 2017. Limnol. Oceanogr. DOI: 10.1002/lno.10511.
This product can be sold containing ProClin if requested.Additional information (application): This product can be sold containing ProClin if requested
in bis-tris gel systems PsbB protein migrates between 40-45 kDa - Background
-
Background: PsbB (CP47) is a chlorophyll-binding protein located in the membrane, where it serves as the core antenna of Photosystem II.
- Product Citations
-
Selected references: Collombat et al. (2025). Arabidopsis conditional photosynthesis mutants abc1k1 and var2 accumulate partially processed thylakoid preproteins and are defective in chloroplast biogenesis. Commun Biol . 2025 Jan 22;8(1):111. doi: 10.1038/s42003-025-07497-y.
Sulli et al. (2023). Generation and physiological characterization of genome‑edited Nicotiana benthamiana plants containing zeaxanthin as the only leaf xanthophyll. Planta . 2023 Oct 5;258(5):93. doi: 10.1007/s00425-023-04248-3.
Zhao et al. (2024). Psb28 protein is indispensable for stable accumulation of PSII core complexes in Arabidopsis.Plant J. 2024 May 26. doi: 10.1111/tpj.16844.
Nagy et al. (2023). Photoautotrophic and sustained H2 production by the pgr5 mutant of Chlamydomonas reinhardtii in simulated daily light conditions. International Journal of Hydrogen Energy Volume 53, 31 January 2024, Pages 760-769.
Vidal-Meireles, et al. (2023)The lifetime of the oxygen-evolving complex subunit PSBO depends on light intensity and carbon availability in Chlamydomonas. Plant Cell Environ. 2023;46(2):422-439. doi:10.1111/pce.14483
Miernicka et al. (2022) The Adjustment Strategy of Venus Flytrap Photosynthetic Apparatus to UV-A Radiation. Cells. 2022;11(19):3030. Published 2022 Sep 27. doi:10.3390/cells11193032
Konert et al (2022). High-light-inducible proteins HliA and HliB: pigment binding and protein-protein interactions. Photosynth Res. 2022 Jun;152(3):317-332. doi: 10.1007/s11120-022-00904-z. Epub 2022 Feb 26. PMID: 35218444.
Guardini et al. (2022). Loss of a single chlorophyll in CP29 triggers re-organization of the Photosystem II supramolecular assembly. Biochim Biophys Acta Bioenerg. 2022 Jun 1;1863(5):148555. doi: 10.1016/j.bbabio.2022.148555. Epub 2022 Apr 2. PMID: 35378087.
Xiong et al. (2022) a chloroplast nucleoid protein of bacterial origin linking chloroplast transcriptional and translational machineries, is required for proper chloroplast gene expression in Arabidopsis thaliana. Nucleic Acids Res. 2022 Jun 23;50(12):6715-34. doi: 10.1093/nar/gkac501. Epub ahead of print. PMID: 35736138; PMCID: PMC9262611.
Beckova et al. (2022). Photosystem II antenna modules CP43 and CP47 do not form a stable 'no reaction centre complex' in the cyanobacterium Synechocystis sp. PCC 6803. Photosynth Res. 2022 Jan 11. doi: 10.1007/s11120-022-00896-w. Epub ahead of print. PMID: 35015206.
Cecchin et al (2021) LPA2 protein is involved in photosystem II assembly in Chlamydomonas reinhardtii. Plant J. 2021 Jul 4. doi: 10.1111/tpj.15405. Epub ahead of print. PMID: 34218480.
Li et al. (2021). Physiological responses of Skeletonema costatum to the interactions of seawater acidification and the combination of photoperiod and temperature. Biogeosciences, 18, 1439–1449, 2021.
Kamea et al. (2021). Substitution of deoxycholate with the amphiphilic polymer amphipol A8-35 improves the stability of large protein complexes during native electrophoresis. Plant Cell Physiol. 2021 Jan 5:pcaa165. doi: 10.1093/pcp/pcaa165. Epub ahead of print. PMID: 33399873.
Aso et al. (2021). Unique peripheral antennas in the photosystems of the streptophyte alga Mesostigma viride. Plant Cell Physiol. 2021 Jan 8:pcaa172. doi: 10.1093/pcp/pcaa172. Epub ahead of print. PMID: 33416834.
Trinugroho et al. (2020). Chlorophyll F Synthesis by a Super-Rogue Photosystem II Complex. Nat Plants , 6 (3), 238-244
Dong et al. (2020). Plastid ribosomal protein LPE2 is involved in photosynthesis and the response to C/N balance in Arabidopsis thaliana. J Integr Plant Biol. 2020 Jan 15. doi: 10.1111/jipb.12907.
Furukawa et al. (2019). Formation of a PSI–PSII megacomplex containing LHCSR and PsbS in the moss Physcomitrella patens. J Plant Res https://doi.org/10.1007/s10265-019-01138-2
Gonzaga Heredia-Martinez et al. (2018). Chloroplast damage induced by the inhibition of fatty acid synthesis triggers autophagy in Chlamydomonas. Plant Physiol, Sept. 2018.
Patil et al. (2018). FZL is primarily localized to the inner chloroplast membrane however influences thylakoid maintenance. Plant Mol Biol. 2018 Jul;97(4-5):421-433. doi: 10.1007/s11103-018-0748-3.
Bressan et al. (2018). Light harvesting complex I is essential for Photosystem II photoprotection under variable light conditions in Arabidopsis thaliana. Environmental and Experimental Botany Available online 10 March 2018.
Myouga et al. (2018). Stable accumulation of photosystem II requires ONE-HELIX PROTEIN1 (OHP1) of the light harvesting-like family. Plant Physiol. 2018 Feb 1. pii: pp.01782.2017. doi: 10.1104/pp.17.01782.
Schottler et al. (2017). The plastid-encoded PsaI subunit stabilizes photosystem I during leaf senescence in tobacco. J Exp Bot. 2017 Feb 1;68(5):1137-1155. doi: 10.1093/jxb/erx009.
Xing et al. (2017). Deletion of CGLD1 Impairs PSII and Increases Singlet Oxygen Tolerance of Green Alga Chlamydomonas reinhardtii. Front. Plant Sci., 15 December 2017.
Blommaert et al. (2017). Contrasting NPQ dynamics and xanthophyll cycling in a motile and a non-motile intertidal benthic diatom. Limnol. Oceanogr. doi: 10.1002/lno.10511
Gandini et al. (2017). The transporter SynPAM71 is located in the plasma membrane and thylakoids, and mediates manganese tolerance in Synechocystis PCC6803. New Phytol. 2017 Mar 20. doi: 10.1111/nph.14526.
Hu et al. (2017). The SUFBC2 D Complex is Required for the Biogenesis of All Major Classes of Plastid Fe-S Proteins. Plant J. 2017 Jan 19. doi: 10.1111/tpj.13483.
Fan et al. (2016). Proteome Analyses Using iTRAQ Labeling Reveal Critical Mechanisms in Alternate Bearing Malus prunifolia. J Proteome Res. 2016 Oct 7;15(10):3602-3616.
Fristedt et al. (2015). The thylakoid membrane protein CGL160 supports CF1CF0 ATP synthase accumulation in Arabidopsis thaliana. PLoS One. 2015 Apr 2;10(4):e0121658. doi: 10.1371/journal.pone.0121658.
Armbruster et al. (2014). Ion antiport accelerates photosynthetic acclimation in fluctuating light environments. Nat Commun. 2014 Nov 13;5:5439. doi: 10.1038/ncomms6439 - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts - Reviews:
-
Marco Schottkowski | 2014-02-18Worked very good to estimate PSII amounts and visualize PSII subcomplexes on two-dimensional PAGE using Synechocystis protein extracts.Yu Qing-Bo | 2009-05-07This antibody works very well in our lab, when it was used to detect the accumulation of photosynthetic protein in Arabidopsis


