1

Anti-PsaB | PSI-B core subunit of photosystem I
AS10 695 | Clonality: Polyclonal | Host: Rabbit | Reactivity: [global antibody] for higher plants, algae, cyanobacteria
Benefits of using this antibody
- Product Info
-
Immunogen: KLH-conjugated synthetic peptide derived from known PsaB sequences including Arabidopsis thaliana UniProt: P56767, TAIR: AtCg00340
Host: Rabbit Clonality: Polyclonal Purity: Antigen affinity purified in PBS pH 7.4 Format: Lyophilized Quantity: 50 µg Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Blue Native PAGE (BN-PAGE), Western blot (WB) Recommended dilution: 1 : 1000 (BN-PAGE), (WB) Expected | apparent MW: 82.7 | 55-60 kDa - Reactivity
-
Confirmed reactivity: Arabidopsis thaliana, Brassica napus, Brassica rapa, Bryopsis corticulans, Echinola crus-galli, Hordeum vulgare, Lolium perenne, Neochloris oleoabundans (chlorophyta), Mesembryanthemum crystallinum, Mesostigma viride, Nicotiana tabacum, Phaeodactylum tricornutum, Pisum sativum, Setaria viridis, Solanum lycopersicum, Synechococcus sp. PCC7942, Synechocystis sp. PCC 6803, Triticum aestivum, Ulva prolifera, Zea mays
Predicted reactivity: Algae, Aloysia triphylla, Beta vulgaris, Borago officinalis, Brachypodium distachyon, Cannabis sativa, Cercidiphyllum japonicum, Citrus x limon, Cyanobacteria, Exbucklandia populnea, Gunnera maniCata, Kalanchoe laciniata, Lagenaria siceraria, Lippia origanoides, Lippia alba, Indocalamus sinicus, Manihot esculenta, Morus notabilis, Medicago truncatula, Monsonia emarginata, Mytilaria laosensis, Geranium endressii, Glycine max, Glycine soja, Lotus japonicus, Oryza sativa, Pandanus utilis, Panax ginseng, Parnassia laxmannii , Pelargonium cotyledonis, Pennisetum americanum, Phaseolus pachyrrhizoides, Phaseolus lunatus, Phaseolus vulgaris, Phyla dulcis,, Pinus thunbergii, Populus trichocarpa, Ribes fasciculatum, Rhodoleia championii, Rhyticaryum macrocarpum, Salvia miltiorrhiza, Setaria italica, Solanum tuberosum, Spinacia oleracea, Triticum sp., Vigna angularis, Vitis vinifera
Species of your interest not listed? Contact usNot reactive in: Chlamydomonas reinhardtii, dinoflagellate
- Application Examples
-

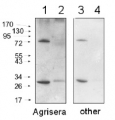
0,5 µg (lanes 1-4) or 1,0 µg (lanes 5-8) of chlorophyll from Pisum sativum (1 and 5), Zea mays, mesophyll (2 and 6) and bundle sheath (3 and 7), Echinochloa crus-galli, mesophyll (4 and 8)chloroplasts extracted with 0.4 M sorbitol, 50 mM Hepes NaOH, pH 7.8, 10 mM NaCl, 5 mM MgCl2 and 2 mM EDTA were loaded to lanes. Samples were denatured with Laemmli buffer at 75 0C for 5 min and were separated on 12% SDS-PAGE, and blotted 30 min to PVDF using wet transfer. Blot was blocked with 5% milk for 2h at room temperature (RT) with agitation. Blot was incubated in the primary antibody Anti-PsaB AS10 695 (LOT 2303) at a dilution of 1: 1000 in 1% milk in TBS-T overnight at 40C with agitation. The antibody solution was decanted, and the blot was washed 4 times for 5 min in TBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG HRP conjugated, from Agrisera, AS09 602, LOT 2304) diluted to 1: 20000 in 1 % milk in TBS-T for 1h at RT with agitation. The blot was washed 5 times for 5 min in TBS-T and 2 times for 5 min in TBS, and developed for 1 min with 1.25 mM luminol, 0.198 mM coumaric acid and 0.009% H2O2 in 0.1 M Tris- HCl, pH 8.5. Exposure time in ChemiDoc System was 83 seconds.
Courtesy Dr. Wiola Wasilewska-Dąbrowska, Warsaw University, Poland
Samples:
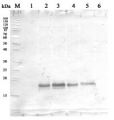
Marker: Blue prestained standard (New England Biolabs)-
0.25 µg of Arabidopsis thaliana thylakoids
-
0.50 µg of Arabidopsis thaliana thylakoids
-
0.75 µg of Arabidopsis thaliana thylakoids
-
1.0 µg of Arabidopsis thaliana thylakoids
Wild type Arabidopsis thaliana (ecotype Colombia) thylakoids were isolated according to Sirpiö et al (2011, Methods Mol Biol. 2011; 775:19-30) Samples were denatured at room temperature (RT), separated on 12 % SDS-PAGE with 6 M urea and blotted 1h to a PVDF membrane (0.45 µm) using a semi-dry transfer. Blot was blocked with 5% milk in TBS for 1h at RT with slow agitation and incubated in primary antibody at a dilution of 1: 3 000 overnight at 4°C with slow agitation in 1 % milk/TTBS. The blot was rinsed briefly once, then washed twice for 10 min with TTBS at RT with agitation. The blot was incubated in secondary antibody (Agrisera anti-rabbit IgG horse radish peroxidase conjugated) in 1% milk/TTBS for 1-2 hours at RT with slow agitation, washed as above and incubated for 5 min with ECL according to the manufacturers’ instructions. Exposure time was 30 seconds.
Courtesy Virpi Paakkarinen, University of Turku, Department of Life Technologies, Molecular Plant Biology Unit, Finland.jpg)
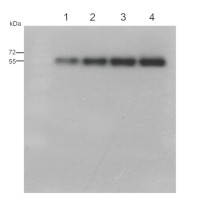
5 µg of total protein from (1) Arabidopsis thaliana leaf extract, (2) Synechococcus sp. PCC 7942 , (3) Hordeum vulgare leaf extract, (4) Physcomitrella patens, (5) Pisum sativum, (6) Zea may were extracted with Agrisera Protein Extraction Buffer PEB and separated on 4-12% NuPage (Invitrogen) LDS-PAGE and blotted 1h to nitrocellulose OSMONICS. Blots were blocked immediately following transfer in 2% blocking reagent in 20 mM Tris, 137 mM sodium chloride pH 7.6 with 0.1% (v/v) Tween-20 (TBS-T) for 1h at room temperature with agitation. Blots were incubated in the primary antibody at a dilution of 1: 10 000 for 1h at room temperature with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at room temperature with agitation. Blots were incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, from Agrisera, AS09 602) diluted to 1:50 000 in 2% blocking solution for 1h at room temperature with agitation. The blots were washed as above and developed for 5 min with che,iluminescent detection reagent according to the manufacturers instructions. Images of the blots were obtained using a CCD imager (FluorSMax, Bio-Rad) and Quantity One software (Bio-Rad). Exposure time was 30 seconds.
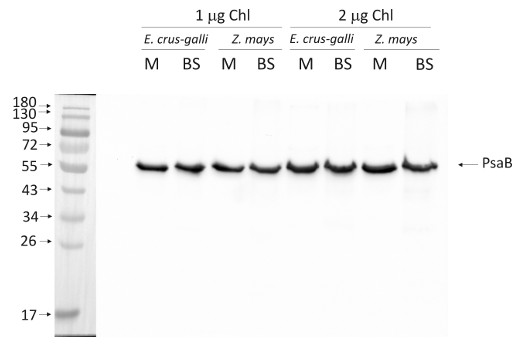
1.0 or 2.0 µg of chlorophyll from mesophyll (M) and bundle sheath (BS) thylakoids of Zea mays and Echinochloa crus-galli extracted with 0.4 M sorbitol, 50 mM Hepes NaOH, pH 7.8, 10 mM NaCl, 5 mM MgCl2 and 2 mM EDTA were loaded to lanes. Samples were denatured with Laemmli buffer at 75 °C for 5 min and were separated on 12% SDS-PAGE and blotted 30 min to PVDF using wet transfer. Blot was blocked with 5% milk for 2h at room temperature (RT) with agitation. Blot was incubated in the primary antibody at a dilution of 1: 1000 overnight at 4°C with agitation in 1% milk in TBS-T. The antibody solution was decanted and the blot was washed 4 times for 5 min in TBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG HRP conjugated, from Agrisera, AS09 602, Lot 1808) diluted to 1:25 000 in 1 % milk in TBS-T for 1h at RT with agitation. The blot was washed 5 times for 5 min in TBS-T and 2 times for 5 min in TBS, and developed for 1 min with 1.25 mM luminol, 0.198 mM coumaric acid and 0.009% H2O2 in 0.1 M Tris- HCl, pH 8.5. Exposure time in ChemiDoc System was 60 seconds.
Courtesy Dr. Wiola Wasilewska-Dąbrowska, Warsaw University, PolandApplication examples: 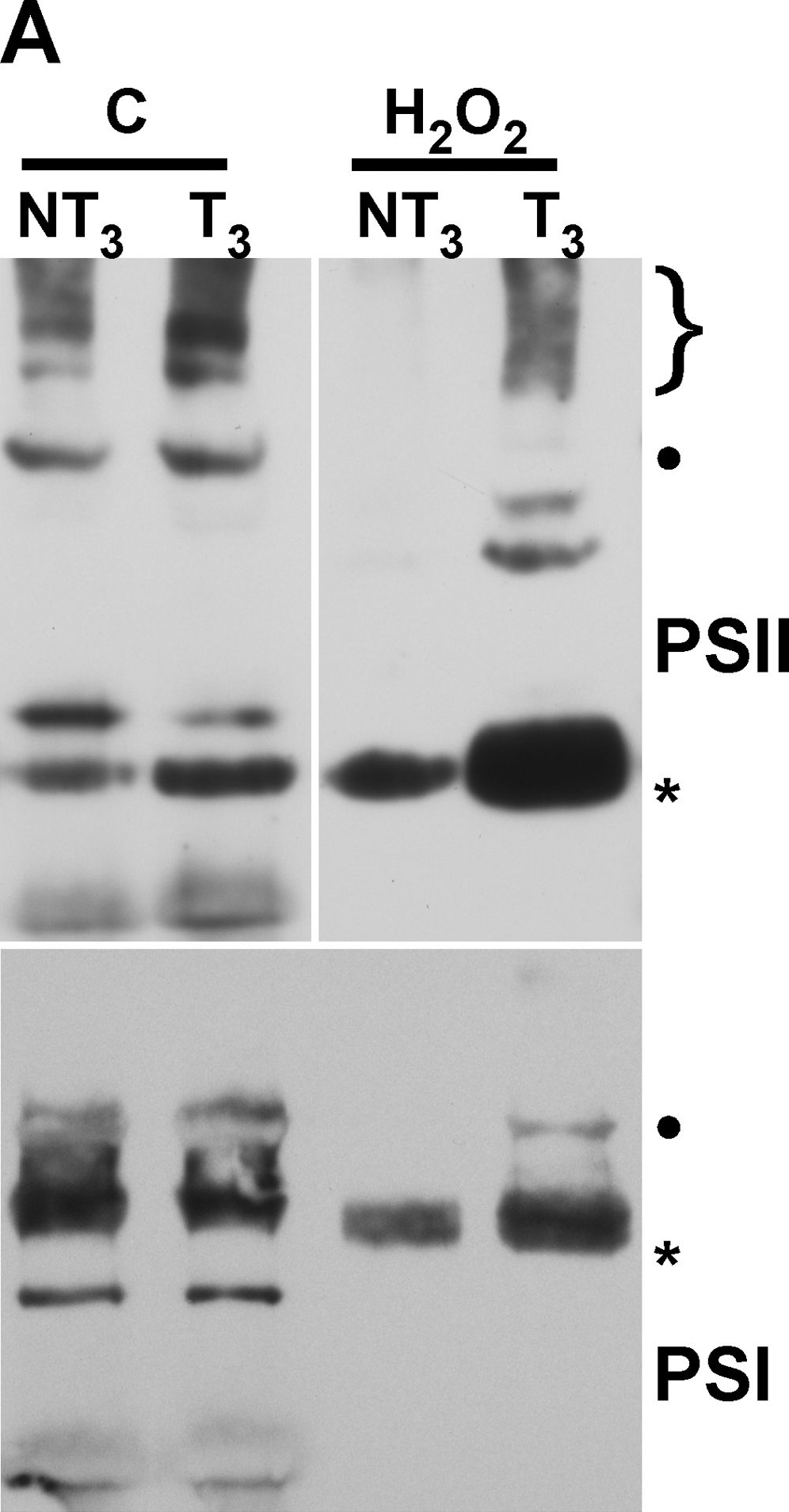
Reactant: Nicotiana tabacum (Common tobacco)
Application: Western Blotting
Pudmed ID: 23227265
Journal: PLoS One
Figure Number: 4A
Published Date: 2012-12-12
First Author: Almoguera, C., Prieto-Dapena, P., et al.
Impact Factor: 2.942
Open PublicationMembrane protein complexes of PSII and PSI survive in the 35S:A9 seedlings.(A) Western detection of complexes after the H2O2 treatments. The PSII complexes (top) separated by BN-PAGE were detected using anti-D1 (DE-loop) antibodies at 1/5,000 dilution. The PSI complexes (bottom) were detected using anti-PsaB antibodies at 1/4,000 dilution. PSII symbols: •, and the bracket on top respectively mark the dimeric PSII complex, and the PSII-LHCII super-complexes. The asterisk marks the CVII (CP43-less PSII monomer) complex. PSI symbols: • marks the PSI-LHCI super-complex that co-migrates in our gel system with the dimeric PSII complex; the asterisk marks the PSI monomer. (B) The PSII complexes also withstand drastic dehydration. The thylakoid samples were analyzed immediately (0 h) after the dehydration treatment (DT), and following rehydration for 16 h, DT (16 h). In this case the complexes were detected using anti-D1 (C-terminal) antibodies at 1/15,000 dilution. An additional PSII complex mentioned in the text is indicated: CV (**, PSII monomer).
Reactant: Synechocystis
Application: Western Blotting
Pudmed ID: 24476911
Journal: Nucleic Acids Res
Figure Number: 6C
Published Date: 2014-04-01
First Author: Gunnelius, L., Hakkila, K., et al.
Impact Factor: 16.476
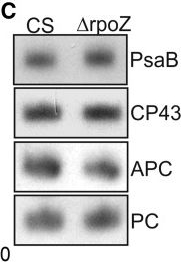
Open PublicationPhotosynthetic properties of ?rpoZ. (A) Light-saturated photosynthetic activity of a 1 ml of culture (OD730 = 1) of CS (white bar) and ?rpoZ (black bar) in standard conditions. Each bar represents an average of three independent biological replicates, and the error bars denote SE. (B) Fluorescence at 77 K was measured using 440-nm light that excites Chl. The data were normalized by dividing with the height of the PSI emission peak at 723 nm. (C) Total proteins were isolated, separated with SDS-PAGE and the amounts of PSI reaction center protein PsaB, PSII core protein CP43 and the phycobilisome proteins allophycocyanin (APC) and phycocyanin (PC) were measured by western blotting. The protein contents of PsaB, CP43, ACP and PC were 99 ± 6%, 97 ± 7, 103 ± 7% and 99 ± 4, respectively, in ?rpoZ of that measured in CS.
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 25835989
Journal: PLoS One
Figure Number: 7A
Published Date: 2015-04-04
First Author: Fristedt, R., Martins, N. F., et al.
Impact Factor: 2.942
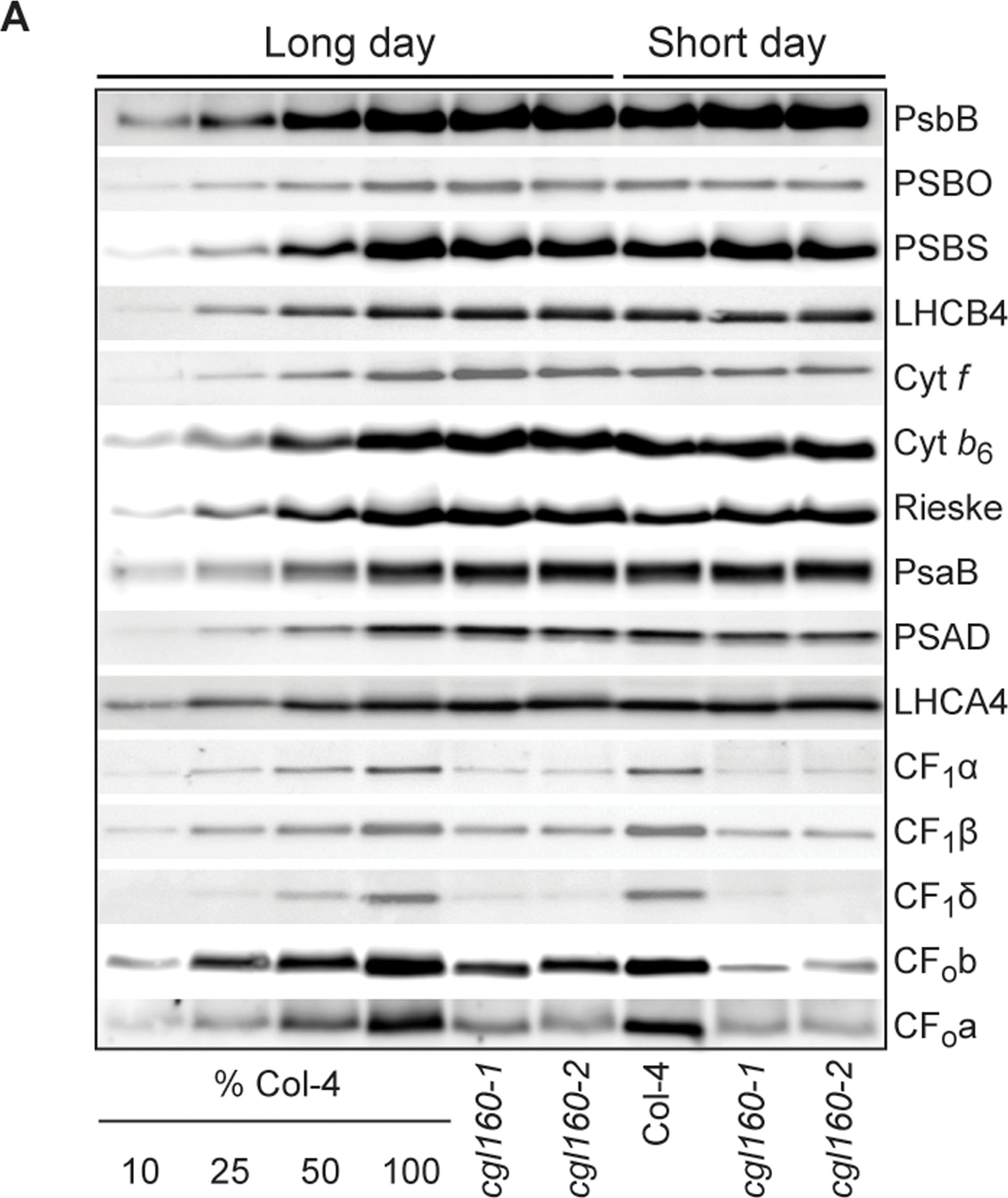
Open PublicationAltered protein accumulation and stability of the chloroplast ATP synthase in the cgl160 mutant visualized by immunoblotting.A. Immunoblots with antibodies against essential subunits of the photosynthetic protein complexes of wild-type (Col-4) Arabidopsis and the two cgl160 T-DNA insertion lines grown under long-day and short-day conditions. Isolated thylakoid membranes were used, and equal amounts of chlorophyll were loaded onto the SDS-PAGE gel. For approximate quantification, wild-type samples from long-day plants were diluted to 10%, 25% and 50%, respectively. Accumulation of PSII was probed with antibodies against PsbB and PSBO. Additionally, the PSBS protein involved in NPQ and the minor PSII antenna protein LHCB4 were probed. Accumulation of the cytochrome b6f complex was probed with antibodies against the essential subunits PetA (cytochrome f), PetB (cytochrome b6), and PETC (Rieske protein). Accumulation of PSI was probed with antibodies against the reaction center subunit PsaB and the stromal ridge subunit PsaD. ATP synthase accumulation was probed with antibodies against the CF1 subunits AtpA (CF1?), AtpB (CF1?) and AtpD (CF1?) and antibodies against the CF0 subunits AtpF (CF0b) and AtpI (CF0a). B. Loading difference estimation for immunoblotting CF1 between wild type and cgl160-1. To obtain similar immunoblotting signal three times more (15 ?g protein) was needed for cgl160-1 compared to wild type (5 ?g protein). C. Maintenance of CF1 was measured by incubating leaves from wild type and cgl160-1 in solution containing the plastid protein synthesis inhibitor chloramphenicol for the indicated time points. Protein extract was isolated and separated by SDS-PAGE, immunoblotted and probed with specific antibodies against CF1 and LHCB2.1. Three times more protein was loaded from the mutant to obtain equal level of CF1 immunoblotting signal, as specified in B.
Reactant: Nicotiana tabacum (Common tobacco)
Application: Western Blotting
Pudmed ID: 28180288
Journal: J Exp Bot
Figure Number: 5A
Published Date: 2017-02-01
First Author: Schöttler, M. A., Thiele, W., et al.
Impact Factor: 6.088
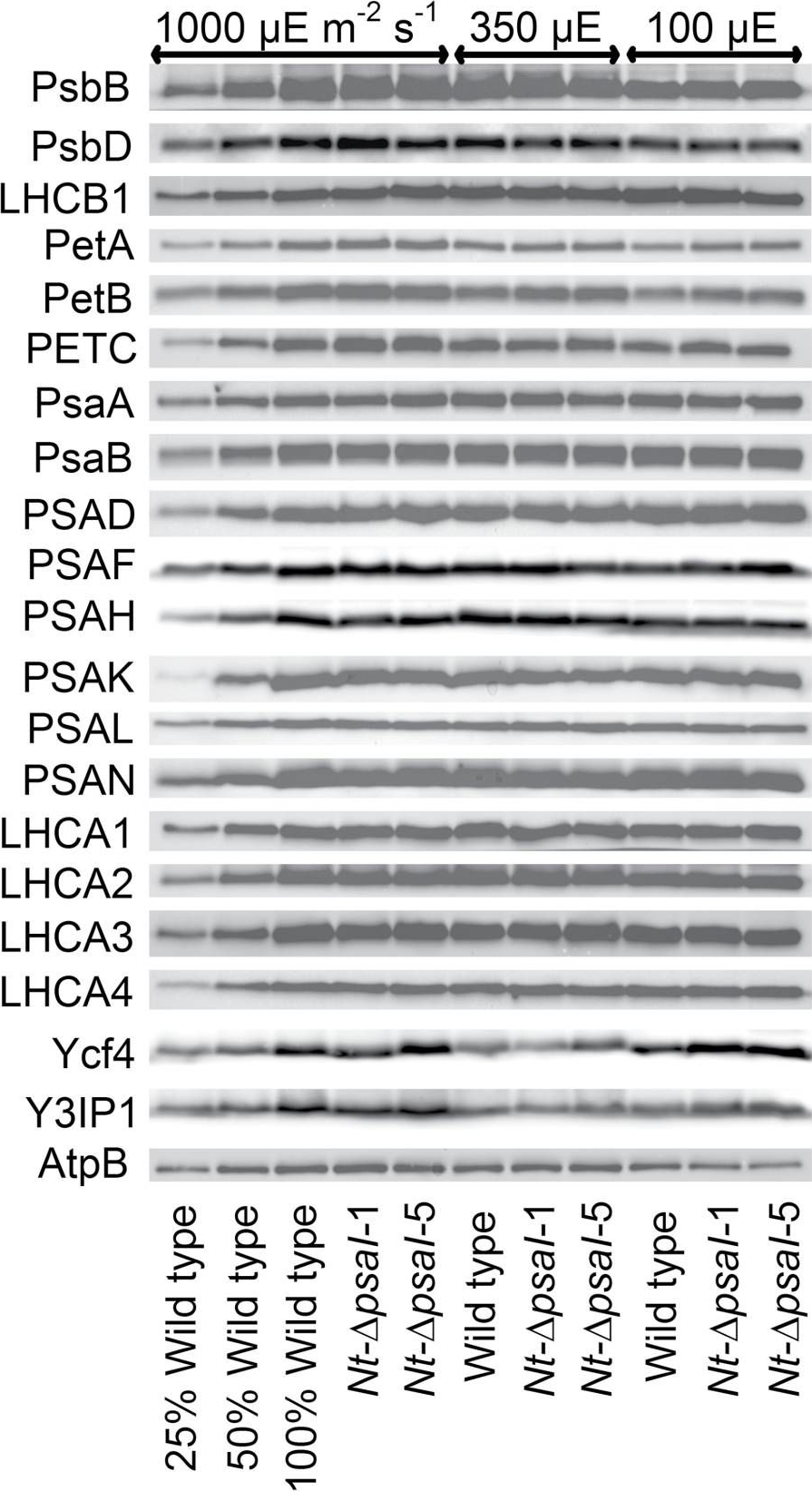
Open PublicationImmunoblot analysis of photosynthetic complex accumulation in wild-type tobacco and the two ?psaI lines grown under low, intermediate, and high-light conditions. Because the accumulation of most tested proteins was highest under high-light conditions, lanes one to three contain samples diluted to 25%, 50%, and a 100% sample of wild-type tobacco grown under high-light conditions, to allow for semi-quantitative determination of changes in protein abundance. Lanes four and five contain the two transplastomic lines grown at 1000 µE m?2 s?1. Lanes six to eight contain wild-type tobacco and the mutants grown at intermediate light intensities, and lanes nine to eleven contain samples grown at low light intensities. For PSII, the accumulation of the essential subunits PsbB (CP43) and PsbD (D2) and the LHCB1 antenna protein were determined, while for the cytochrome b6f complex, the accumulation of the essential redox-active subunits PetA (cytochrome f), PetB (cytochrome b6), and PETC (Rieske FeS protein) was tested. AtpB was probed as an essential subunit of the chloroplast ATP. For PSI, in addition to the three essential plastome-encoded subunits PsaA, PsaB, and PsaC, the accumulation of the nuclear-encoded subunits PSAD, PSAH, PSAK, PSAL, and PSAN and of the four LHCI proteins (LHCA1, LHCA2, LHCA3, LHCA4) was determined. Finally, we examined the accumulation of Ycf4, the chloroplast-encoded PSI-biogenesis factor encoded in the same operon as PsaI, and the nuclear-encoded assembly factor Y3IP1.
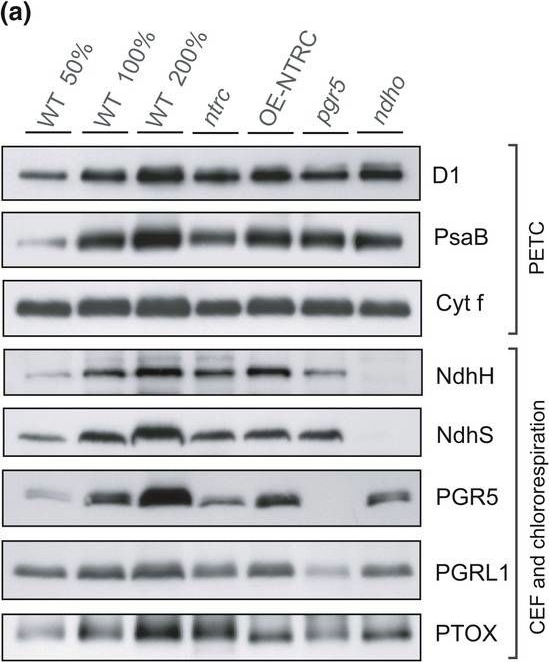
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 31245694
Journal: Plant Direct
Figure Number: 3A
Published Date: 2018-11-01
First Author: Nikkanen, L., Toivola, J., et al.
Impact Factor: None
Open PublicationContent of proteins functioning in the photosynthetic electron transfer chain (PETC), cyclic electron flow (CEF), and chlororespiration in WT, ntrc, OE?NTRC, pgr5, and ndho. (a) Representative immunoblots showing the content of D1, PsaB, Cyt f, NdhH, NdhS, PGR5, PGRL1, and PTOX. Appropriate amount of thylakoid extract (based on protein content) was separated with SDS?PAGE and probed with specific antibodies. Equal loading was confirmed by protein staining with Li?Cor Revert Total Protein Stain. (b) Relative content of proteins in mutant lines as percentage of WT. The numbers represent the average protein content ±SE in three to five biological replicates. The quantified values were normalized to the total protein content in the sample determined with Li?Cor Revert Total Protein Stain. Statistically significant differences to WT according to Student's t tests (p < 0.05) are marked with *
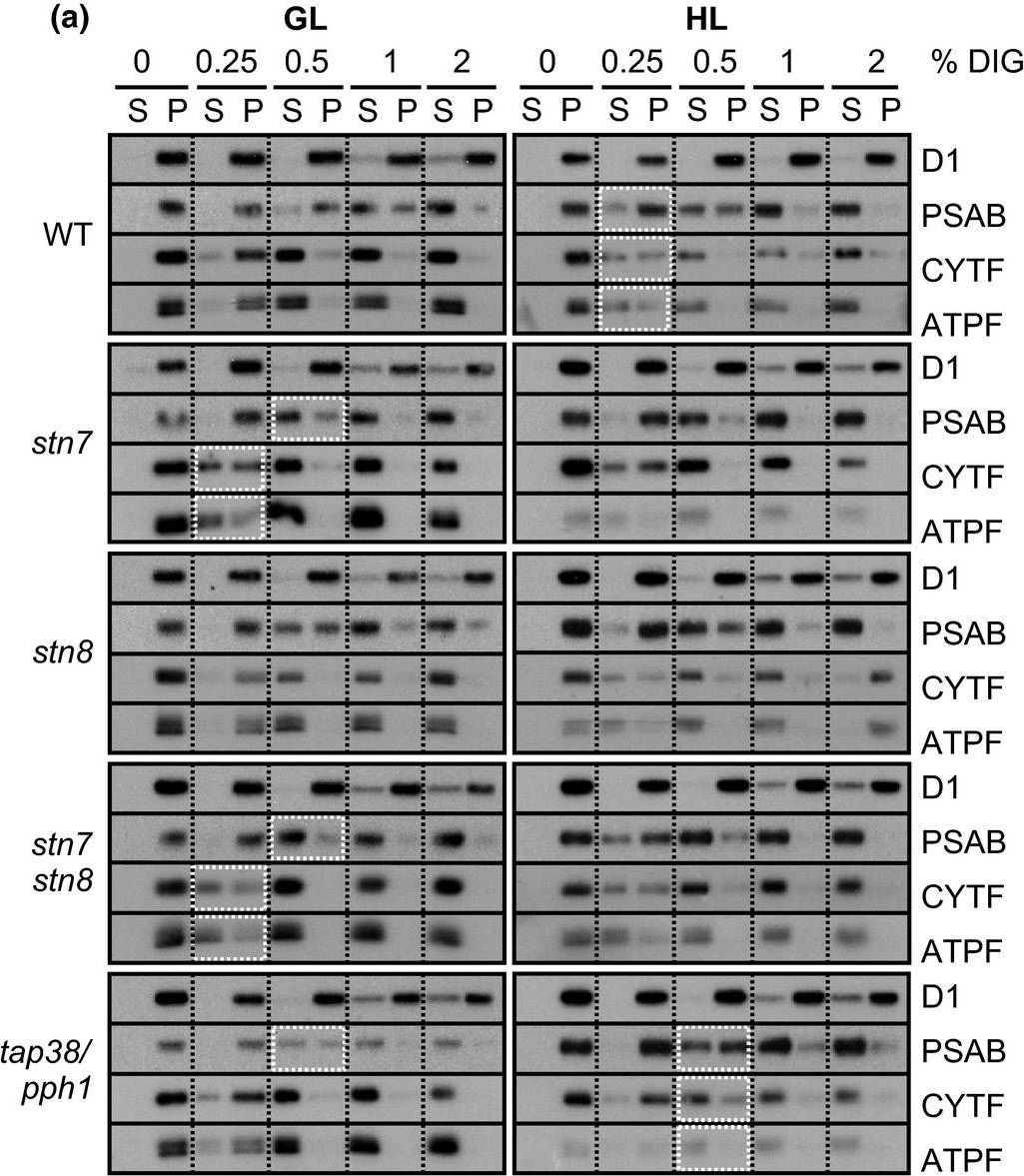
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 31245706
Journal: Plant Direct
Figure Number: 5A
Published Date: 2018-02-01
First Author: Rantala, S. & Tikkanen, M.
Impact Factor: None
Open PublicationStepwise detachment of photosynthetic protein complexes from the thylakoid membrane of WT, stn7, stn8, stn7stn8, and tap38/pph1. Thylakoid membranes of WT, stn7, stn8, stn7stn8, and tap38/pph1 plants harvested from moderate growth light (GL, 120 ?mol photons m?2 s?1) or after high light illumination (HL, 600 ?mol photons m?2 s?1 for 2 hr) were isolated and solubilized with 0%, 0.25%, 0.5%, 1%, and 2% DIG. Equal volumes of the soluble supernatant (S) and insoluble pellet (P) fractions were loaded and separated in SDS?PAGE followed by immunodetection of (a) proteins D1, PSAB, CYTF, and ATPF representing the protein complexes PSII, PSI, LHCII, Cyt b6f, and ATP synthase, respectively, as well as (b) proteins LHCB1, P?LHCB1, LHCB2, P?LHCB2, and LHCB3, representing the different subunits of the LHCII complexes. The differences in mutants with respect to WT as well as the differences in WT in response to Hl are marked with white boxes. Representative data from three different biological replicates are shown
-
- Additional Information
-
Additional information (application): This product can be sold containing ProClin if requested - Background
-
Background: Photosystem I (PSI) of chloroplasts is a multisubunit membrane-protein complex that catalyzes the electron transfer from the reduced plastocyanin (or cytochrome c6) in the thylakoid lumen to the oxidized ferredoxin (or flavodoxin) in the chloroplast stroma. PsaB is a core protein of PSI complex. Synonymes: Photosystem I P700 chlorophyll a apoprotein A2, PSI-B
- Product Citations
-
Selected references: Popova et al. (2025). Differential Photosynthetic Response of Tomato Plants—Ailsa Craig and Carotenoid Mutant tangerine—To Low Light Intensity and Low Temperature Treatment. Crops 2025, 5(6), 77; doi.org/10.3390/crops5060077
Woodford et al. (2025). PROTON GRADIENT REGULATION 5 enables efficient C4 photosynthesis under fluctuating light. Plant Physiol. 2025 Sep 29:kiaf466.doi: 10.1093/plphys/kiaf466.
Pilarska et al. (2025). Salinity-induced changes in the PSII/LHCII phosphorylation and organization of the photosynthetic protein complexes in the halophyte Mesembryanthemum crystallinum L. Journal of Plant Physiology, 10 July 2025, 154567.
Wójtowicz et al. (2025). Shrink or expand? Just relax! Bidirectional grana structural dynamics as early light-induced regulator of photosynthesis. New Phytol . 2025 Jun;246(6):2580-2596. doi: 10.1111/nph.70175.
Xie et al, (2024). LpY3IP1 Enhances the drought and salt tolerance of perennial ryegrass by protecting the photosynthetic apparatus. Scientia Horticulturae Volume 338, 1 December 2024, 113645.
Tiwari et al. (2024). Differential FeS cluster photodamage plays a critical role in regulating excess electron flow through photosystem I. Nat Plants. 2024 Oct;10(10):1592-1603. doi: 10.1038/s41477-024-01780-2.
Ermakova et al. (2024). Chloroplast NADH dehydrogenase-like complex-mediated cyclic electron flow is the main electron transport route in C4 bundle sheath cells. New Phytol. 2024 Jul 22.doi: 10.1111/nph.19982.
Grebe et al. (2024). Accounting for photosystem I photoinhibition sheds new light on seasonal acclimation strategies of boreal conifers. Journal of Experimental Botany, erae145
Völkner et al. (2024). Evidence for partial functional overlap of KEA and MSL transport proteins in the chloroplast inner envelope of Arabidopsis thaliana. FEBS Lett. 2024 Aug;598(15):1877-1887. doi: 10.1002/1873-3468.14887.
Jiang et al. (2023). Toxic effects of lanthanum (III) on photosynthetic performance of rice seedlings: Combined chlorophyll fluorescence, chloroplast structure and thylakoid membrane protein assessment. Ecotoxicol Environ Saf. 2023 Nov 15:267:115627.doi: 10.1016/j.ecoenv.2023.115627.
Seed priming with NaCl helps to improve tissue tolerance, potassium retention ability of plants, and protects the photosynthetic ability in two different legumes, chickpea and lentil, under salt stress. Planta. 2023 May 8;257(6):111. doi: 10.1007/s00425-023-04150-y.
Cao et al. (2023). An unexpected hydratase synthesizes the green light--absorbing pigment fucoxanthin
Krynicka, et al. (2023) FtsH4 protease controls biogenesis of the PSII complex by dual regulation of high light-inducible proteins. Plant Commun. 2023;4(1):100502. doi:10.1016/j.xplc.2022.100505
Lempiainen et al. (2022) Plants acclimate to Photosystem I photoinhibition by readjusting the photosynthetic machinery. Plant Cell Environ. 2022 Oct;45(10):2954-2971. doi: 10.1111/pce.14400. Epub 2022 Aug 16. PMID: 35916195.
Zhao et al. (2022) Native architecture and acclimation of photosynthetic membranes in a fast-growing cyanobacterium. Plant Physiol. 2022;190(3):1883-1895. doi:10.1093/plphys/kiac372
Shukla et al. (2020). A novel method produces native LHCII aggregates from the photosynthetic membrane revealing their role in non-photochemical quenching. J Biol Chem. 2020 Oct 20:jbc.RA120.016181. doi: 10.1074/jbc.RA120.016181. Epub ahead of print. PMID: 33082138.
Grieco et al. (2020). Adjustment of photosynthetic activity to drought and fluctuating light in wheat. Plant Cell Environ. 2020 Mar 16. doi: 10.1111/pce.13756.
Liu et al. (2020). Acid treatment combined with high light leads to increased removal efficiency of Ulva prolifera. Algal Research,Volume 45, January 2020, 101745
Frede et al. (2019). Light quality-induced changes of carotenoid composition in pak choi Brassica rapa ssp. chinensis. J Photochem Photobiol B. 2019 Apr;193:18-30. doi: 10.1016/j.jphotobiol.2019.02.001.
Lima-Melo et al. (2019). Consequences of photosystem-I damage and repair on photosynthesis and carbon use in Arabidopsis thaliana. Plant J. 2018 Nov 29. doi: 10.1111/tpj.14177.
Koochak et al. (2018). The structural and functional domains of plant thylakoid membranes. Plant J. 2018 Oct 12. doi: 10.1111/tpj.14127. (Blue Native PAGE)
Gao et al. (2018). Effect of green light on the amount and activity of NDH-1–PSI supercomplex in Synechocystis sp. strain PCC 6803. Photosynthetica (2018) 56: 316.
Popova et al. (2018). Differential temperature effects on dissipation of excess light energy and energy partitioning in lut2 mutant of Arabidopsis thaliana under photoinhibitory conditions. Photosynth Res. 2018 May 3. doi: 10.1007/s11120-018-0511-2.
Rantala and Tikkanen et al. (2018). Phosphorylation‐induced lateral rearrangements of thylakoid protein complexes upon light acclimation. Plant Direct Vol. 2, Issue 2.
Wang et al. (2018). iTRAQ-based quantitative proteomics analysis of an immature high-oleic acid near-isogenic line of rapeseed. Molecular Breeding January 2018, 38:2.
Kurkela et al. (2017). Acclimation to High CO2 Requires the ω Subunit of the RNA Polymerase in Synechocystis. Plant Physiol. 2017 May;174(1):172-184. doi: 10.1104/pp.16.01953. Epub 2017 Mar 28.
Schottler et al. (2017). The plastid-encoded PsaI subunit stabilizes photosystem I during leaf senescence in tobacco. J Exp Bot. 2017 Feb 1;68(5):1137-1155. doi: 10.1093/jxb/erx009.
Giovanardi et al. (2017). Higher packing of thylakoid complexes ensures a preserved Photosystem II activity in mixotrophic Neochloris oleoabundans. Algal Research, Volume 25, July 2017, Pages 322-332.
Jusovic et al. (2017). Photosynthetic Responses of a Wheat Mutant (Rht-B1c) with Altered DELLA Proteins to Salt Stress. Journal of Plant Growth Regulation, pp1-12.
Georg et al. (2017). Acclimation of Oxygenic Photosynthesis to Iron Starvation Is Controlled by the sRNA IsaR1. Curr Biol. 2017 May 22;27(10):1425-1436.e7. doi: 10.1016/j.cub.2017.04.010. (Synechocystis 6803 substrain PCC-M)
Tyuereva et al. (2017). The absence of chlorophyll b affects lateral mobility of photosynthetic complexes and lipids in grana membranes of Arabidopsis and barley chlorina mutants. Photosynth Res. 2017 Apr 5. doi: 10.1007/s11120-017-0376-9. (Hordeum vulgare, western blot)
Gandini et al. (2017). The transporter SynPAM71 is located in the plasma membrane and thylakoids, and mediates manganese tolerance in Synechocystis PCC6803. New Phytol. 2017 Mar 20. doi: 10.1111/nph.14526.
Nath et al. (2016). A Nitrogen-Fixing Subunit Essential for Accumulating 4Fe-4S-Containing Photosystem I Core Proteins. Plant Physiol. 2016 Dec;172(4):2459-2470. Epub 2016 Oct 26.
Fristedt et al. (2015). The thylakoid membrane protein CGL160 supports CF1CF0 ATP synthase accumulation in Arabidopsis thaliana. PLoS One. 2015 Apr 2;10(4):e0121658. doi: 10.1371/journal.pone.0121658.
Suorsa et al. (2015). Light acclimation involves dynamic re-organisation of the pigment-protein megacomplexes in non-appressed thylakoid domains. Plant J. 2015 Aug 29. doi: 10.1111/tpj.13004.
Grieco et al. (2015). Light-harvesting II antenna trimers connect energetically the entire photosynthetic machinery - including both photosystems II and I. Biochim Biophys Acta. 2015 Jun-Jul;1847(6-7):607-19. doi: 10.1016/j.bbabio.2015.03.004. Epub 2015 Apr 3.
Subramanyam et al. (2014). Structural and functional changes of PSI-LHCI supercomplexes of Chlamydomonas reinhardtii cells grown under high salt conditions. Planta. 2010 Mar;231(4):913-22.
Qin et al. (2014). Isolation and characterization of a PSI-LHCI super-complex and its sub-complexes from a siphonaceous marine green alga, Bryopsis Corticulans. Photosynth Res. 2014 Sep 12.
Mustila et al. (2014). The bacterial-type [4Fe–4S] ferredoxin 7 has a regulatory function under photooxidative stress conditions in the cyanobacterium Synechocystis sp. PCC 6803. BBA- Bioenergetics, April 2014. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Oxygenic photosynthesis poster by prof. Govindjee and Dr. Shevela
Z-scheme of photosynthetic electron transport by prof. Govindjee and Dr. Björn and Dr. Shevela - Reviews:
-
Monika Ostaszewska | 2021-08-16Good detection on A.thaliana thylakoid membrane fraction samples containing 1 ug of chlorophyll per well on 10% gel (primary antibody concentration 1:1000).Katya Georgieva | 2019-06-24Worked very well on thylakoid proteins from Haberlea rhodopensis. We used 3 ug chlorophyll per lane, 1:1000 dilution of PsaB, 1:25000 dilution of 2nd antibody, ECL detection of WB. Reference: Mihailova G, Büchel C, Dietzel L, Georgieva K (2016) Desiccation induced changes in photosynthesis related proteins of shade and sun Haberlea rhodopensis plants. Compt. Rend Bulg. Acad. Sci. 69(1), 37-44| 2014-12-20We obtained very good results using whole cell exctracts of Phaeodactylum tricornutum. Here are the details: 4 µg of total protein from Phaeodactylum tricornutum were loaded on a 13% SDS-PAGE and blotted 1.5 h to nitrocellulose. Blots were blocked immediately following transfer in 5% milk-TBS-T for 1h at room temperature with agitation. Blots were incubated in the primary antibody at a dilution of 1:2000 overnight at 4 oC with agitation. The antibody solution was decanted and the blot was rinsed briefly, then washed 3 times for 5 min in TBS-T at room temperature with agitation. Blots were incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated) diluted to 1:10 000 in TBS-T for 1h at room temperature with agitation. The blots were washed as above and developed 6 sec with ECL detection reagent. Images of the blots were obtained using a CCD imager (Chemidock MP Imaging, Bio-Rad).Maya Velitchkova | 2014-04-30We obtain very good results using this antibody with thylakoid membranes from peas and tomato and PSI particles from spinach.| 2011-01-14Works fairly well for pumpkin at 1:500 with 10 ug protien loaded but this dilution is very high. Similar results with Quercus alba and Acer rubrum thylakoids.
Accessories
AS06 172 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. thaliana, C. quitensis Kunt Bartl, C. pumilum, C. reinhardtii, C. zofingiensis, F. vesiculosus, H.vulgare, M. polymorpha, N. oceanica, N. tabacum, O. sativa, P. abies, P. sativum, P. strobus, P. vulgaris, S. oleracea, C.reinhardtii, Synechococcus PCC 7942, Synechocystis PCC 6803, Scenedesmus obliquus, microalgae N. gaditana