1
Anti-PC | Plastocyanin
AS06 141 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. thaliana, B. juncea, H. annuus, L. sativus, M. polymorpha, N. tabacum, O. sativa, P. sativum, S. oleracea, S. tuberosum, Synechocystis sp. PCC6803, Z. mays
compartment marker of chloroplast thylakoid lumen
- Product Info
-
Immunogen: Purified native plastocyanin from Spinacia oleracea UniProt: P00289 Host: Rabbit Clonality: Polyclonal Purity: Serum Format: Lyophilized Quantity: 50 µl Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Immunogold (IG), Western blot (WB) Recommended dilution: 1 : 100 (IG), 1 : 2000 (WB) Expected | apparent MW: 10 kDa
- Reactivity
-
Confirmed reactivity: Arabidops thaliana, Brassica juncea, Chlamydomonas reinhardtii, Heliantus annuus, Hordeum vulgare, Lathyrus sativus, Marchantia polymorpha, Nicotiana tabacum, Oryza sativa, Pisum sativum, Spinacia oleracea, Solanum tuberosum, Synechocystis sp. PCC6803, Zea mays Predicted reactivity: Dicots, Chlamydomonas reinhardtii, Nicotiana benthamiana, Physcomitrium patens, Ricinus communis, Solanum lycopersicum, Suaeda salsa
Species of your interest not listed? Contact usNot reactive in: No confirmed exceptions from predicted reactivity are currently known - Application Examples
-

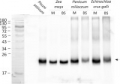
Thylakoid membranes (10 µg of total chlorophyll) extracted freshly from Hordeum vulgare leaves with 100 mM HEPES-KOH (pH 7.5), 0.3 M sorbitol, 2 mM EDTA, and 1mM MgCl2 and denatured with a Laemmli buffer at 80°C for 5 min were separated on 12% SDS-PAGE and blotted 1 h to nitrocellulose (pore size of 0.2 um), using semi-dry transfer. Blot was blocked with 4% milk for 2 h/RT with agitation. Blot was incubated in the primary antibody at a dilution of 1:3000 (PC and PetA, simultaneous western blot detection for both antibodies at the same time) for 1 h/RT with agitation in PBS-T. The antibody solution was decanted and the blot was rinsed briefly, then washed 3 times for 5 min in PBS-T at RT with agitation. Blot was incubated in Agrisera matching secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated) diluted to 1:25000 in for 1 h/RT with agitation. The blot was washed as above and developed for 5 min with chemiluminescent detection reagent according to manufactures recommendations. Exposure time was 30 seconds. Simultaneous western blot detection can be applied if MW of detected proteins differs in min. 20 kDa.
Courtesy Dr. Anja Liszkay, CNRS, France
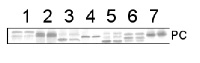
10 µg of total protein from Arabidopsis thaliana (1), Brassica juncea (2), Zea mays (3), Oryza sativa (4), Solamum lycopersicum (5), Nicotiana tabacum (6), Heliantus annuus (7) were separated on SDS-PAGE and blotted to nitrocellulose. Filters were probed with anti-PC antibody (AS06 141, 1:2000). Signal was developed using alkaline phosphatase conjugated secondary antibody. Each sample was run in duplicate. Signal was developed using alkaline conjugated secondary antibody.
This antibody will also work well with HRP-conjugated secondary antibodies, as AS09 602.Application examples: 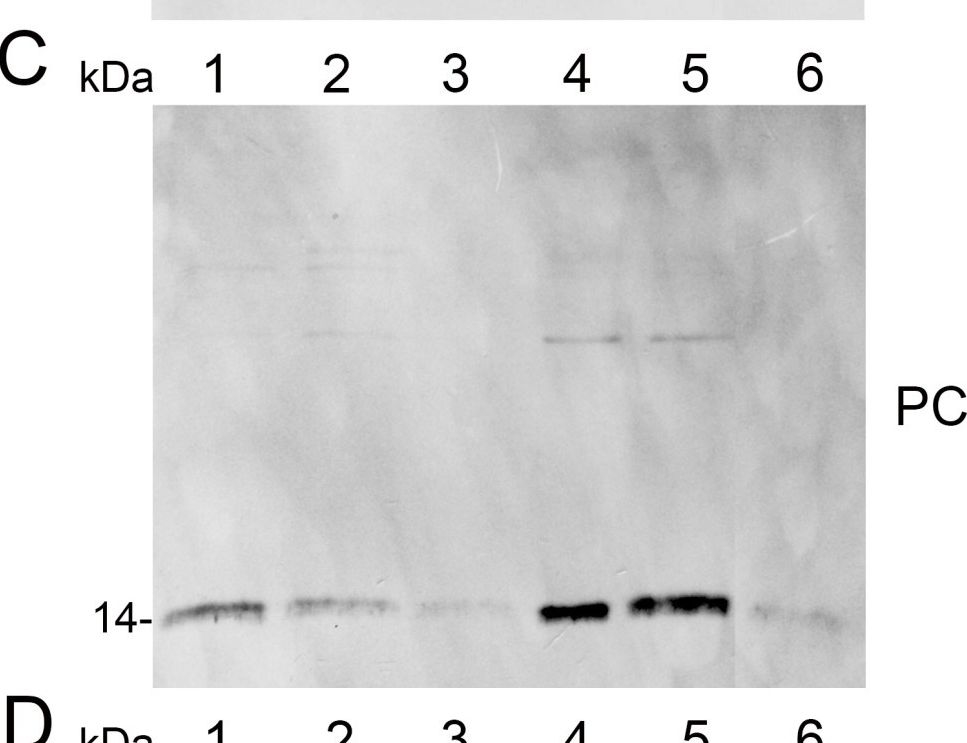
Reactant: Malus domestica (Apple)
Application: Western Blotting
Pudmed ID: 23886449
Journal: Plant Methods
Figure Number: 2C
Published Date: 2013-07-28
First Author: Sikorskaite, S., Rajamäki, M. L., et al.
Impact Factor: 5.139
Open PublicationAnalysis of protein fractions from different steps of nuclei isolation. The protein fractions obtained following the main steps of the procedure used for isolation of nuclei from leaves of apple are shown. Equal amounts of proteins (4 ?g) from each fraction were loaded on the gel. A) Lumenal-binding protein 2 (BiP2), B) histone H3 and C) plastocyanin (PC) were detected with specific antibodies by western blot analysis. D) Coomassie blue staining of the fractionated proteins separated by polyacrylamide gel electrophoresis. Lane 1: crude extract of proteins from homogenized leaf tissue; lane 2: resuspended pellet of the whole cell lysate obtained following treatment with Triton X-100 and centrifugation; lane 3: nuclei collected from the 60% Percoll layer (see nuclear fraction in Figure 1B); lane 4: the interface fraction of 60% Percoll and 2.5 M sucrose layers in the density gradient containing chloroplasts and unbroken cells (see 60% P/2.5 M S interface in Figure 1B); lane 5: sucrose layer of the density gradient (see 2.5 M sucrose layer in Figure 1B); lane 6: nuclei purified by centrifugation on a 35% Percoll cushion.
Reactant: Pisum sativum (Pea)
Application: Western Blotting
Pudmed ID: 33445673
Journal: Int J Mol Sci
Figure Number: 6A
Published Date: 2021-01-12
First Author: Tokarz, K. M., Weso?owski, W., et al.
Impact Factor: 5.542
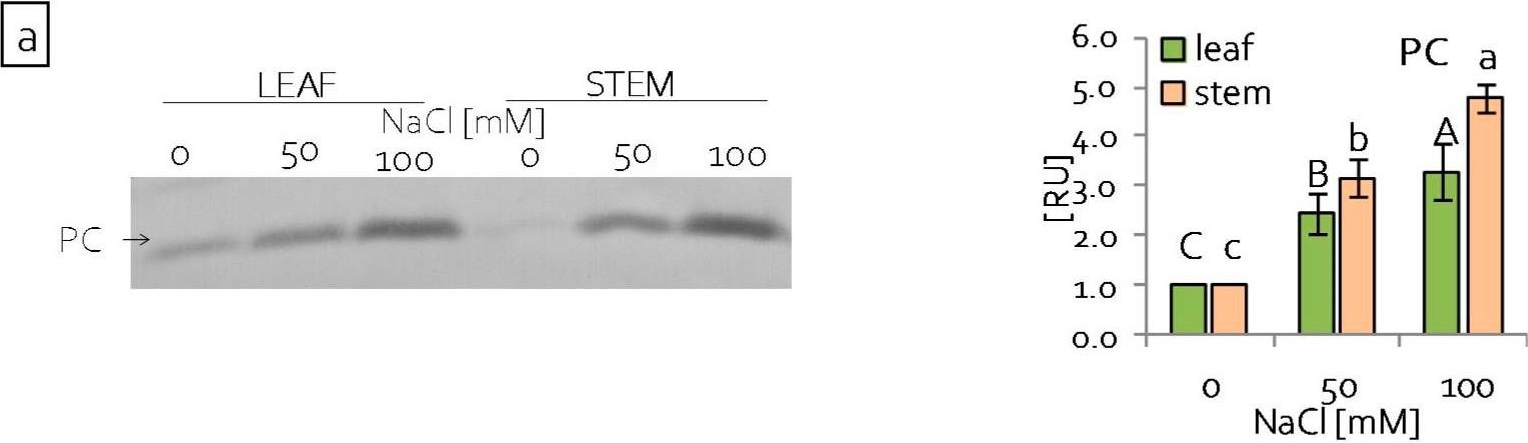
Open PublicationContent of (a) plastocyanin (PC) and (b) large subunit of RuBisCo (RbcL) of grass pea leaf and stem photosynthetic apparatus under salinity; content of proteins expressed as relative units [RU]; different letters—statistically significant differences within each organ (leaf-uppercase, stem-lowercase) at p ? 0.05; (n = 3).
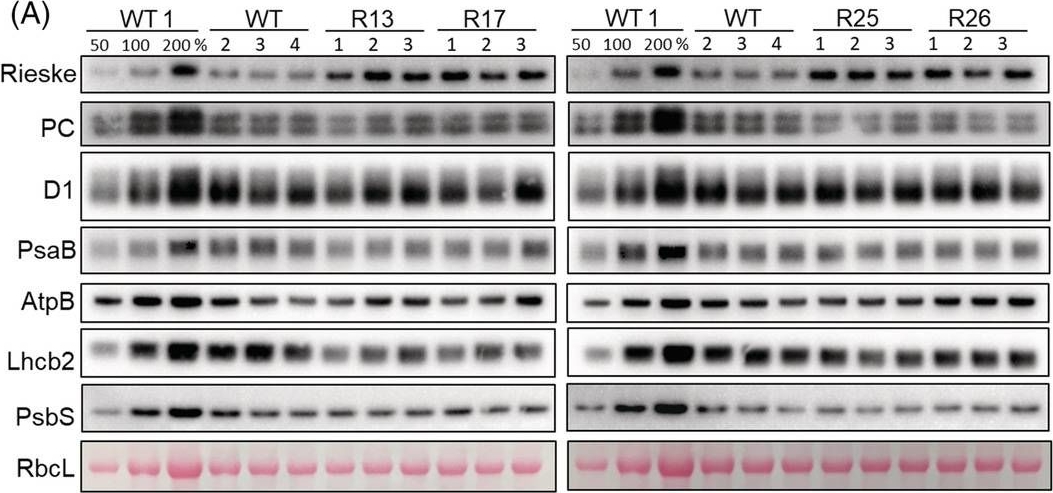
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 36259085
Journal: Physiol Plant
Figure Number: 4A
Published Date: 2022-11-01
First Author: Heyno, E.
Impact Factor: 4.812
Open PublicationAbundance of photosynthetic proteins in leaves of WT and Rieske‐OE plants. (A) Immunodetection of Rieske, plastocyanin (PC), D1 subunit of PSII, PsaB subunit of PSI, AtpB subunit of ATP synthase, Lhcb2 subunit of LHCII and PsbS in leaf protein extracts loaded on leaf area basis. The quantity of the large subunit of Rubisco (RbcL) was estimated from Ponceau stained membranes immediately after transfer. A titration series of one of the WT samples (WT1) was used for relative quantification. (B) Quantification of immunoblots relative to WT. mean ± SE, n = 4 biological replicates for WT, n = 3 for transgenic lines. Asterisks indicate statistically significant differences between transgenic lines and WT (Tukey test, *p < 0.05, **p < 0.01. ***p < 0.001).
- Additional Information
-
Additional information: Cellular [compartment marker] of chloroplast thylakoid lumen
This product can be sold containing ProClin if requested.Additional information (application): Plastocyanin runs abberant due to negative charge at 12-19 kDa on SDS-PAGE depending upon the system used. in 15% gel the protein will run closer to its true MW than in 12% gel. In some cases PC can be very acidic and run at twice of its MW.
PC1 runs closer to 14 kDa while PC2 runs closer to 19 kDa. For good resolution adding fresh DTT to the sample buffer is recommended.
PC2 is generally more abundant and it increases with Cu feeding. PC1 is expressed first after etiolated seedlings are placed in the light. - Background
-
Background: Plastocyanin (PC) is a small Cu protein and a mobile electron carrier in the lumen of the thylkoids. PC interacts with the B/F complex and Photosystem I. Alternative name: DNA-damage-repair/toleration protein DRT112.
- Product Citations
-
Selected references: Wu et al. (2025). Plastocyanin affects photosynthesis and high light acclimation by modulating redox states of electron transport chain in Chlamydomonas reinhardtii. Commun Biol . 2025 Mar 21;8(1):476. doi: 10.1038/s42003-025-07904-4.
Collombat et al. (2025). Arabidopsis conditional photosynthesis mutants abc1k1 and var2 accumulate partially processed thylakoid preproteins and are defective in chloroplast biogenesis. Commun Biol . 2025 Jan 22;8(1):111. doi: 10.1038/s42003-025-07497-y.
Hani and Krieger-Liszkay (2024). Manganese deficiency alters photosynthetic electron transport in Marchantia polymorpha. Elsevier Plant Physiology and Biochemistry Available online 16 August 2024, 109042.
Penzler et al. (2024). A pgr5 suppressor screen uncovers two distinct suppression mechanisms and links cytochrome b6f complex stability to PGR5. Plant Cell. 2024 Mar 27:koae098. doi: 10.1093/plcell/koae098.
Mu et al. (2024). Plastid HSP90C C-terminal extension region plays a regulatory role in chaperone activity and client binding.Plant J. 2024 Jul 5.doi: 10.1111/tpj.16917.
Lian et al. (2023). MicroRNA397 promotes rice flowering by regulating the photorespiration pathway. Plant Physiol. 2023 Nov 23:kiad626. doi: 10.1093/plphys/kiad626.
Hao and Malnoë (2023). A Simple Sonication Method to Isolate the Chloroplast
Lumen in Arabidopsis thaliana.Bio Protoc. 2023 Aug 5; 13(15): e4756.
Naschberger, Mosebach, Tobiasson, et al. (2022) Algal photosystem I dimer and high-resolution model of PSI-plastocyanin complex. Nat Plants. 2022;8(10):1191-1201. doi:10.1038/s41477-022-01253-4
Urban, Rogowski & Romanowska (2022), Crucial role of the PTOX and CET pathways in optimizing ATP synthesis in mesophyll chloroplasts of C3 and C4 plants, Environmental and Experimental Botany, Volume 202, October 2022, 105024, https://doi.org/10.1016/j.envexpbot.2022.105028.
Tokarz et al. (2021). Stem Photosynthesis-A Key Element of Grass Pea (Lathyrus sativus L.) Acclimatisation to Salinity. Int J Mol Sci. 2021 Jan 12;22(2):685. doi: 10.3390/ijms22020685. PMID: 33445673; PMCID: PMC7828162.
Viola et al. (2021) In vivo electron donation from plastocyanin and cytochrome c6 to PSI in Synechocystis sp. PCC6803. Biochim Biophys Acta Bioenerg. 2021 May 15;1862(9):148449. doi: 10.1016/j.bbabio.2021.148449. Epub ahead of print. PMID: 34004195.
Furutani et al. (2021) The difficulty of estimating the electron transport rate at photosystem I. J Plant Res. 2021 Nov 15. doi: 10.1007/s10265-021-01357-6. Epub ahead of print. PMID: 34778922.
Wang et al. (2020) Rerouting of ribosomal proteins into splicing in plant organelles. BioRxiv, DOI: 10.1101/2020.03.03.974766 .
Galvis et al. (2020). H+ transport by K+ EXCHANGE ANTIPORTER3 promotes photosynthesis and growth in chloroplast ATP synthase mutants. Plant Physiol. pp.01561.2019. doi: 10.1104/pp.19.01561.
Simakawa et al. (2020). Near-infrared in Vivo Measurements of Photosystem I and Its Lumenal Electron Donors With a Recently Developed Spectrophotometer. Photosynth Res. , 144 (1), 63-72.
Cha et al. (2019). Arabidopsis GIGANTEA negatively regulates chloroplast biogenesis and resistance to herbicide butafenacil. Plant Cell Rep. 2019 Jul;38(7):793-801. doi: 10.1007/s00299-019-02409-x.
Mermod et al. (2019). SQUAMOSA promoter-binding protein-like 7 mediates copper deficiency response in the presence of high nitrogen in Arabidopsis thaliana. Plant Cell Rep. 2019 May 15. doi: 10.1007/s00299-019-02422-0.
Balyan et al. (2017). Identification of miRNA-mediated drought responsive multi-tiered regulatory network in drought tolerant rice, Nagina 22. Sci Rep. 2017 Nov 13;7(1):15446. doi: 10.1038/s41598-017-15450-1.
Perea-García et al. (2017). Arabidopsis copper transport protein COPT2 participates in the cross talk between iron deficiency responses and low-phosphate signaling. Plant Physiol. 2013 May;162(1):180-94. doi: 10.1104/pp.112.212407.
Yoshida et al. (2016). Hisabori T1.Two distinct redox cascades cooperatively regulate chloroplast functions and sustain plant viability. Proc Natl Acad Sci U S A. 2016 Jul 5;113(27):E3967-76. doi: 10.1073/pnas.1604101113. Epub 2016 Jun 22.
Kropat et al. (2015). Copper economy in Chlamydomonas: Prioritized allocation and reallocation of copper to respiration vs. photosynthesis. Proc Natl Acad Sci U S A. 2015 Feb 2. pii: 201422492.
Sook Seok et al. (2013). AtFKBP16-1, a chloroplast lumenal immunophilin, mediates response to photosynthetic stress by regulating PsaL stability. Physiologia Plantarum, DOI: 10.1111/ppl.12116.
Perera-Garcia et al. (2013). Arabidopsis copper transport protein COPT2 participates in the crosstalk between iron deficiency responses and low phosphate signaling. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts - Reviews:
-
Susanne Braun | 2017-01-24We have used PC antibody with protein extrac from Hordeum vulgare.We got a special bandLoad per well: 15µg total Protein on a 14 % SDS-GelMembrane: NC von Roth, HP40.1Blocken: 5% milk in TBST, 2h, RTPlastocyanin AS06 141: 1:2000 / 1 x TBST, over night, 4°CSecondary antibody : Rabbit anti goat IgG AS 09605 1:50000 in 2% milk / 1 x TBST 1h RT Developing reagent: ECL ( GE Healthcare )1:1:2Exposure time: 1minSidona | 2011-11-16We have used PC antibody with protein extract from Nicotiana and Malus species at dilution 1:6000 and both isoforms of PC could be detected. The same dilution could be reused at least 3 times.Sergi Puig | 2009-05-29We have used your PC antibody with protein extracts from Arabidopsis plants grown with different concentrations of copper in the medium,and we have been able to detect a specific band for Pc and its decrease when copper concentration diminishes, as previously describe.We are satisfied with the quality of the antibody.