1
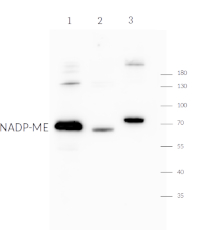
Anti-NADP-ME | NADP-malic enzyme, chloroplastic (dicots/monocots)
AS21 4580 | Clonality: Polyclonal | Host: Rabbit | Reactivity: Flaveria bidentis, Neurachne muelleri , Pisum sativum, Zea mays
- Product Info
-
Immunogen: KLH-conjugated peptide derived from Flaveria trinervia NADP-ME, UniProt: P22178 Host: Rabbit Clonality: Polyclonal Purity: Antigen affinity purified serum, in PBS pH 7.4 Format: Lyophilized Quantity: 50 µg Reconstitution: For reconstitution add 50 µl, of sterile water. Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please, remember to spin tubes briefly prior to opening them to avoid any losses that might occur from lyophilized material adhering to the cap or sides of the tubes. Tested applications: Western blot (WB) Recommended dilution: 1 : 5000 (WB) Expected | apparent MW: 71.5 | 65 kDa (due to N-terminal processing) - Reactivity
-
Confirmed reactivity: Flaveria bidentis, Neurachne alopecuroidea, Neurachne muelleri, Oryza sativa, Pisum sativum, Zea mays
Predicted reactivity: Arabidopsis thaliana, Beta vulgaris subsp. Vulgaris, Citrus clementina,Cynara cardunculus var. ScolymusKalanchoe fedtschenkoi, Elaeis guineensis, Elaeis guineensis,Lactuca sativa, Flaveria robusta, Flaveria pringlei, Flaveria floridana, Flaveria brownii, Flaveria palmeri, Flaveria linearis, Glycine max,Glycine soja,Helianthus annuus, Hibiscus syriacus, Lupinus angustifolius, Musa acuminata subsp. Malaccensis, Vigna angularis, Phtheirospermum japonicum, Sesamum indicum, Spatholobus suberectus,Vigna unguiculata, Vigna radiata var. Radiata, Vigna angularis
Monocots: Hordeum vulgare, Oryza sativa, Setaria viridis, Zea mays
Species of your interest not listed? Contact usNot reactive in: No confirmed exceptions from predicted reactivity are currently known - Application Examples
-

Samples:
Flaveria bidentis whole leaf extract - 1
Zea mays whole leaf extract - 2
Neurachne muelleri whole leaf extract - 35 ug/well of total protein extracted from leaves of species listed above, were extracted in 50 mM HEPES, pH 7.1, 1% (w/v) polyvinylpolypyrrolidone, 1 mM EDTA, 10 mM DTT, 5 mM MgCl2, 1 mM PMSF and denatured with 0.5 M Tris-HCl pH 6.8, 10% (w/v) SDS, 10% (v/v) glycerol, 0.001% (w/v) bromophenol blue at 90°C for 5 min. Samples were separated on 10% SDS-PAG and blotted for 1 h to nitrocellulose (pore size of 0.45 um), using semi-dry transfer. Blot was blocked with 5% (w/v) skim milk powder in TBS-T for 1 h at RT with agitation. Blot was incubated in the primary antibody (rabbit anti NADP-ME, chloroplastic, affinity purified serum, AS21 4580 lot 2206) at a dilution of 1: 5 000 in TBS-T ON at 4°C with agitation. The antibody solution was decanted, and the blot was rinsed briefly twice, then washed 3 times for 5 min each time in TBS-T at RT with agitation. Blot was incubated in matching secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated) diluted to 1: 25 000 in for 1 h at room RT with agitation. The blot was washed as above and developed with a following chemiluminescent detection reagent: Agrisera AgriseraBright. Exposure time was 30 seconds or 5 minutes.
Courtesy of Prof Martha Ludwig, University of Western Australia, Australia

Protein was extracted from leaves of Arabidopsis thaliana, Oryza sativa and Neurachne alopecuroidea in protein extraction buffer (50 mM HEPES, pH 7.1, 1% (w/v) polyvinylpolypyrrolidone, 1 mM EDTA, 10 mM DTT, 5 mM MgCl2, 1 mM PMSF) and flash frozen at -80°C. Aliquots of protein extract were thawed and denatured in SDS loading buffer at 90°C for 5 minutes. 15 ug of total protein was loaded per well and separated on 10 % SDS-PAGE and blotted for 1.5 h to nitrocellulose (pore size of 0.45 um), using semi-dry transfer. Blot was blocked with 5 % milk in TBS-T for 1h/RT or with agitation. Blot was incubated in primary at a dilution of 1: 5 000 in TBS-T ON/4°C with agitation. The antibody solution was decanted, and the blot was rinsed briefly twice, then washed 3 times for 5 min in TBS-T at RT with agitation. Blot was incubated in matching secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated) diluted to 1: 25 000 in TBS-T for 1h/RT with agitation. The blot was washed as above and developed with a following chemiluminescent detection reagent: Agrisera AgriseraBright. Exposure time was 1 minute.
Although C3 plants make several homologues of NADP-ME, they do not express high levels of the homologue that is plentiful in the C4 species. This explains the weak labelling even when a relatively high amount of leaf protein extract is on the blots.Courtesy of Prof Martha Ludwig, University of Western Australia, Australia
- Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Agrisera Educational Poster Collection - Reviews:
-
This product doesn't have any reviews.
Accessories
AS09 458 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. comosus, A. thaliana, C. ciliaris, C. gayana, C. velia, Ch. quinoa, H. vulgare, J. curcas, K. prostrata, L. fusca, Lupinus sp. , M. maximus, M. crystallinum, N. tabacum, O. sativa, P. antidotale, P. coloratum, P. strobus, Saccharum spp. hybrid clone C91-301, S. lanata, S. laricifolia, S. bicolor, Synechocystis PCC 6803, Phaeodactylum tricornutum (strain CCAP 1055/1), T. weissfloggi, Z. mays, Z. muelleri
Benefits of using this antibody
50% discount on matching standard/positive control
AS09 458S PEPC | Phosphoenolpyruvate carboxylase positive control/quantitation standard
Use promotional code: Stand50
AS07 241 | Clonality: Polyclonal | Host: Rabbit | Reactivity: [global antibody] for plants Ananas comosus, Miscantus giganteus, Mouse, Nannochloropsis oceanica, Oryza sativa, Panicum maximum,, Panicum virgatum, Phaseolus vulgaris, Saccharum spp. hybrid clone C91-301, Spartina alterniflora, Spartina patens, Zea mays
Benefits of using this antibody

