1
Anti-Lhcb3 | LHCII type III chlorophyll a/b-binding protein
AS01 002 | Clonality: Polyclonal | Host: Rabbit | Reactivity: Photosynthetic eukaryotes including A. thaliana, A. hypogaea, Ch. vulgaris, H. vulgare, L. esculentum (Solanum lycopersicon), M. crystallinum, N. tabacum, O. sativa, P. sativum, P. patens, Prasinoderma sp., Pyramimonas sp., P. vulgaris, S. oleracea, T. aestivum, Triticale, Z. mays
- Product Info
-
Immunogen: BSA-conjugated synthetic peptide derived from a highly conserved sequence of Lhcb3 proteins from angiosperms (monocots and dicots) and gymnosperms, including Arabidopsis thaliana Lhcb3 UniProt: Q9S7M0,TAIR:AT5G54270. This sequence is highly conserved even in Ginko biloba and one of the major LHCII-forms of Physcomitrella patens.
Host: Rabbit Clonality: Polyclonal Purity: Immunogen affinity purified serum in PBS pH 7.4 Format: Lyophilized Quantity: 50 µg Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Western blot (WB) Recommended dilution: 1 : 2000 (WB) Expected | apparent MW: 28.7 | 26 kDa for Arabidopsis thaliana
- Reactivity
-
Confirmed reactivity: Arabidopsis thaliana, Arachis hypogaea, Chlorella vulgaris, Cucumis sativa, Dactylis glomeRata, Hordeum vulgare, Lycopersicon esculentum (Solanum lycopersicon), Mesembryanthemum crystallinum, Nicotiana tabacum, Oryza sativa, Pisum sativum, Phaseolus vulgaris, Physcomirella patens, Prasinoderma sp., Pyramimonas sp., Spinacia oleracea, Triticum aestivum, Triticale, Zea may, Verbascum lychnitis Predicted reactivity: Cucumis melo, Dicots, Gymnosperms, Mosses
Species of your interest not listed? Contact usNot reactive in: No confirmed exceptions from predicted reactivity are currently known - Application Examples
-

Samples:
1 - 10 ug of 4 days old of wild-type Arabidopsis thaliana seedlings extract
2 - 5 ug of 4 days old of wild-type Arabidopsis thaliana seedlings extract
3- 10 ug of 4 days old of gun5-1 mutant seedlings extract
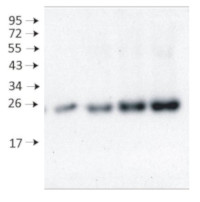
10 µg/well of total protein extracted from fresh 4 days old of Arabidopsis thaliana whole seedling. Exact buffer components were: (50 mM Tris–HCl pH 7.5, 10% glycerol, 150 mM NaCl, 10 mM MgCl2, 5 mM EDTA, 5 mM DTT, 0.5% (v/v) Triton X-100, and 1 × protease inhibitors) and denatured with 4X SDS sample loading buffer (200 mM Tris-HCl (pH 6.8). 8% SDS (sodium dodecyl sulfate). 0.4% Bromophenol blue. 40% glycerol) at 95°C 10 min. Samples were separated in the RT on 15 % SDS-PAGE and blotted for 0.5 h to PVDF (pore size of 0.2 um), using: semi-dry at room temperature. Blot was blocked with 5 % milk for: 1h/RT with agitation. Blot was incubated in the primary antibody at a dilution of 1: 2 000 at 4°C with agitation overnight. The antibody solution was decanted, and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at RT with agitation. Blot was incubated in matching secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated AS09 602 Agrisera) diluted to 1: 50 000 for 2 h/RT with agitation. The blot was washed as above and developed with a following chemiluminescent detection reagent: AS16 ECL-N-10 AgriseraBright (mid picogram). Exposure time was 5 seconds.Courtesy of Dr .Duorong Xu, LMU München, Germany

From 1 μg to 8 μg of chlorophyll from Arabidopsis thaliana chloroplasts extracted with 0.4 M sorbitol, 50 mM Hepes NaOH, pH 7.8, 10 mM NaCl, 5 mM MgCl 2 and 2 mM EDTA were loaded to lanes. Samples were denatured with Laemmli buffer at 75 0 C for 5 min and were separated on 12% SDS-PAGE, and blotted 30 min to PVDF using wet transfer. Blot was blocked with 5% milk for 2h at room temperature (RT) with agitation. Blot was incubated in the primary antibody Anti-Lhcb3 (LOT 1901) at a dilution of 1: 2000 in 1% milk in TBS-T overnight at 4 0 C with agitation. The antibody solution was decanted and the blot was washed 4 times for 5 min in TBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG HRP conjugated, from Agrisera, AS09 602) diluted to 1:20 000 in 1 % milk in TBS-T for 1h at RT with agitation. The blot was washed 5 times for 5 min in TBS-T and 2 times for 5 min in TBS, and developed for 1 min with 1.25 mM luminol, 0.198 mM coumaric acid and 0.009% H 2O2 in 0.1 M Tris- HCl, pH 8.5. Exposure time in ChemiDoc System was 240 seconds.
Courtesy of Dr. Wioleta Wasilewska-Dębowska, University of Warsaw, Poland
Application examples: 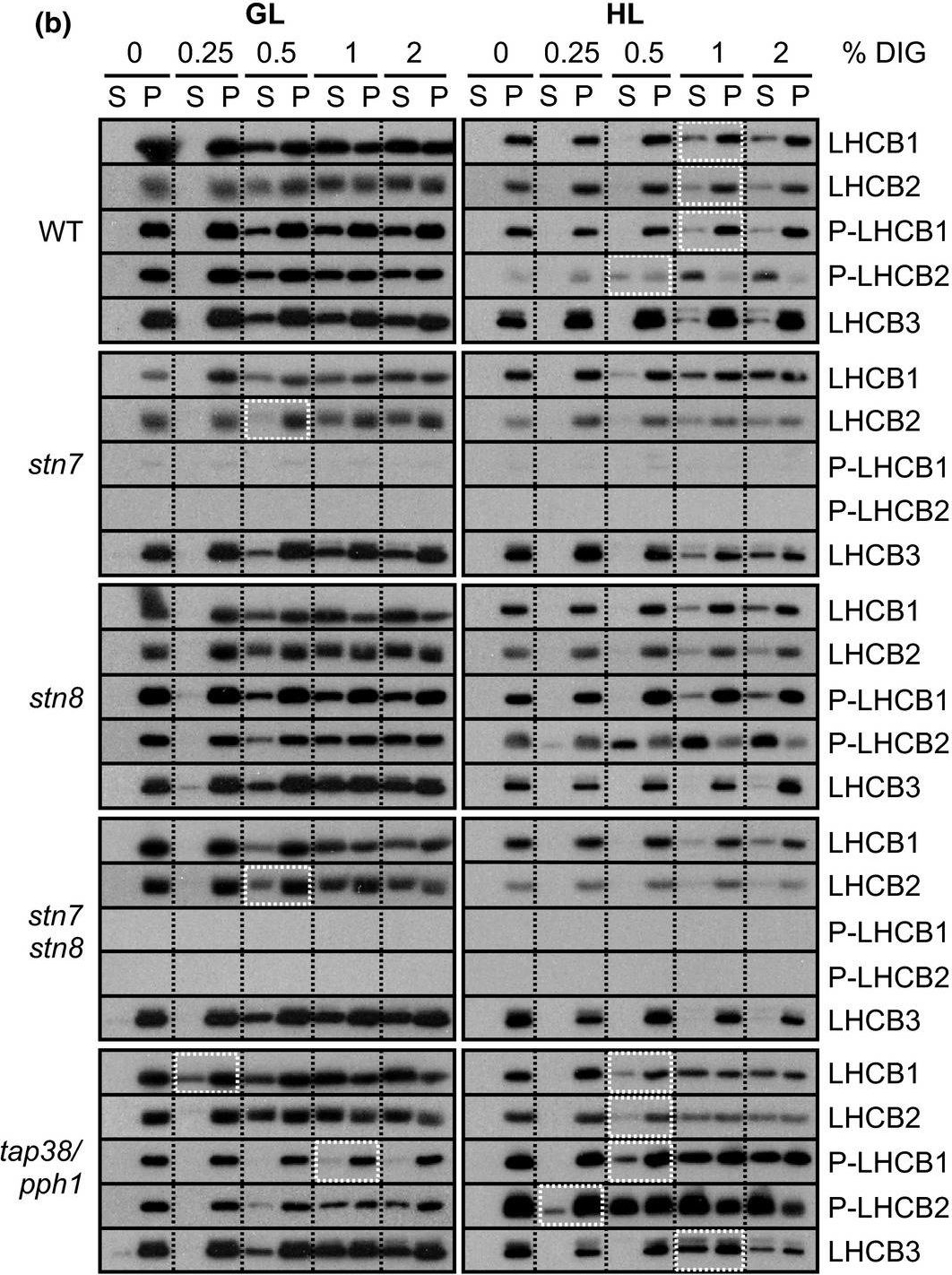
Reactant: Mus musculus (House mouse)
Application: Western Blotting
Pudmed ID: 31245706
Journal: Plant Direct
Figure Number: 5B
Published Date: 2018-02-01
First Author: Rantala, S. & Tikkanen, M.
Impact Factor: None
Open PublicationStepwise detachment of photosynthetic protein complexes from the thylakoid membrane of WT, stn7, stn8, stn7stn8, and tap38/pph1. Thylakoid membranes of WT, stn7, stn8, stn7stn8, and tap38/pph1 plants harvested from moderate growth light (GL, 120 ?mol photons m?2 s?1) or after high light illumination (HL, 600 ?mol photons m?2 s?1 for 2 hr) were isolated and solubilized with 0%, 0.25%, 0.5%, 1%, and 2% DIG. Equal volumes of the soluble supernatant (S) and insoluble pellet (P) fractions were loaded and separated in SDS?PAGE followed by immunodetection of (a) proteins D1, PSAB, CYTF, and ATPF representing the protein complexes PSII, PSI, LHCII, Cyt b6f, and ATP synthase, respectively, as well as (b) proteins LHCB1, P?LHCB1, LHCB2, P?LHCB2, and LHCB3, representing the different subunits of the LHCII complexes. The differences in mutants with respect to WT as well as the differences in WT in response to Hl are marked with white boxes. Representative data from three different biological replicates are shown
- Additional Information
-
Additional information: Antibody format is a total IgG fraction, which means that it is a pool of polyclonal antibodies obtained by purification of serum on Protein G, not on a specific antigen column.
Additional information (application): Protein is processed into mature form (Jansson 1999). - Background
-
Background: The major light-harvesting antenna complex II (LHCII) in photsynthetic eukaryotes is located in the thylakoid membrane of the chloroplast. It is a heterotrimeric complex formed by up to 3 different individual subtypes of chlorophyll a/b-binding proteins: Lhcb1, Lhcb2, and Lhcb3. While Lhcb1 and Lhcb2 are quite similar and regularily present in multiple gene-copies, the Lhcb3 protein differs in pigment-composition and molecular size and often is coded by only a single gene. Lhcb3 seems not to be present in the mobile LHCII trimers involved in state 1-state 2 transitions.
A molecular characterisation of the LHCII proteins can be found in Caffarri et al. (2004) A Look within LHCII: Differential Analysis of the Lhcb1−3 Complexes Building the Major Trimeric Antenna Complex of Higher-Plant Photosynthesis. Biochemistry 43 (29): 9467–9476. - Product Citations
-
Selected references: Ciesielska et al. (2024). S2P2-the chloroplast-located intramembrane protease and its impact on the stoichiometry and functioning of the photosynthetic apparatus of A. thaliana. Front Plant Sci. 2024 Mar 15:15:1372318. doi: 10.3389/fpls.2024.1372318.
Wu et al (2023) Disruption of LEAF LESION MIMIC 4 affects ABA synthesis and ROS accumulation in rice.
Leister et al (2023) An ancient metabolite damage-repair system sustains photosynthesis in plants.
von Bismarck, et al (2023). Light acclimation interacts with thylakoid ion transport to govern the dynamics of photosynthesis in Arabidopsis. New Phytol. 2023;237(1):160-176. doi:10.1111/nph.18534
Li et al. (2022) The CDC48 complex mediates ubiquitin-dependent degradation of intra-chloroplast proteins in plants. Cell Rep. 2022 Apr 12;39(2):110664. doi: 10.1016/j.celrep.2022.110664. PMID: 35417702.
Bru, Steen, Park, et al. (2022) The major trimeric antenna complexes serve as a site for qH-energy dissipation in plants. J Biol Chem. 2022;298(11):102519. doi:10.1016/j.jbc.2022.102522
Ivanov et al. (2022) The decreased PG content of pgp1 inhibits PSI photochemistry and limits reaction center and light-harvesting polypeptide accumulation in response to cold acclimation. Planta 255, 36 (2022). https://doi.org/10.1007/s00425-022-03819-0
von Bismarck et al. (2021) Light acclimation interacts with thylakoid ion transport to govern the dynamics of photosynthesis. Research Square; 2021. DOI: 10.21203/rs.3.rs-948381/v1.
Wu et al. (2021). Formation of light-harvesting complex (LHC) II aggregates from LHCII-PSI-LHCI complexes in rice plants under high light. J Exp Bot. 2021 May 3:erab188. doi: 10.1093/jxb/erab188. Epub ahead of print. PMID: 33939808.
Wojtowicz et al. (2020). Compensation Mechanism of the Photosynthetic Apparatus in Arabidopsis thaliana ch1 Mutants. Int J Mol Sci. 2020 Dec 28;22(1):221. doi: 10.3390/ijms22010221. PMID: 33379339; PMCID: PMC7794896.
Koh et al. (2019). Heterologous synthesis of chlorophyll b in Nannochloropsis salina enhances growth and lipid production by increasing photosynthetic efficiency. Biotechnol Biofuels. 2019 May 14;12:122. doi: 10.1186/s13068-019-1462-3. eCollection 2019.
Furukawa et al. (2019). Formation of a PSI–PSII megacomplex containing LHCSR and PsbS in the moss Physcomitrella patens. J Plant Res https://doi.org/10.1007/s10265-019-01138-2.
Lv et al. (2019). Uncoupled Expression of Nuclear and Plastid Photosynthesis-Associated Genes Contributes to Cell Death in a Lesion Mimic Mutant. Plant Cell. 2019 Jan;31(1):210-230. doi: 10.1105/tpc.18.00813.
Rogowski et al. (2019). Photosynthesis and organization of maize mesophyll and bundle sheath thylakoids of plants grown in various light intensities. Environmental and Experimental Botany Volume 162, June 2019, Pages 72-86.
Mao et al. (2018). Comparison on Photosynthesis and Antioxidant Defense Systems in Wheat with Different Ploidy Levels and Octoploid Triticale. Int J Mol Sci. 2018 Oct 2;19(10). pii: E3006. doi: 10.3390/ijms19103006.
Rantala and Tikkanen et al. (2018). Phosphorylation‐induced lateral rearrangements of thylakoid protein complexes upon light acclimation. Plant Direct Vol. 2, Issue 2.
Myouga et al. (2018). Stable accumulation of photosystem II requires ONE-HELIX PROTEIN1 (OHP1) of the light harvesting-like family. Plant Physiol. 2018 Feb 1. pii: pp.01782.2017. doi: 10.1104/pp.17.01782.
Shin et al. (2017), Complementation of a mutation in CpSRP43 causing partial truncation of light-harvesting chlorophyll antenna in Chlorella vulgaris. Sci Rep. 2017 Dec 20;7(1):17929. doi:10.1038/s41598-017-18221-0.
Tyuereva et al. (2017). The absence of chlorophyll b affects lateral mobility of photosynthetic complexes and lipids in grana membranes of Arabidopsis and barley chlorina mutants. Photosynth Res. 2017 Apr 5. doi: 10.1007/s11120-017-0376-9. (Hordeum vulgare, western blot)
Yang-Er Chen et al. (2017). Responses of photosystem II and antioxidative systems to high light and high temperature co-stress in wheat. J. of Exp. Botany, Volume 135, March 2017, Pages 45–55.
Rozpadek et al. (2015). The fungal endophyte Epichloë typhina improves photosynthesis efficiency of its host orchard grass (Dactylis glomerata). Planta. 2015 Jun 10.
Yokono et al. (2015). A megacomplex composed of both photosystem reaction centres in higher plants. Nat Commun. 2015 Mar 26;6:6675. doi: 10.1038/ncomms7675.
Yao et al. (2015). Ultraviolet-B protection of ascorbate and tocopherol in plants related with their function on the stability on carotenoid and phenylpropanoid compounds. Plant Physiology and Biochemistry Volume 90, May 2015, Pages 23–31.
Kunugi et al. (2016). Evolution of Green Plants Accompanied Changes in Light-Harvesting Systems. Plant Cell Physiol. 2016 Apr 6. pii: pcw071. Qin et al. (2014). Isolation and characterization of a PSI-LHCI super-complex and its sub-complexes from a siphonaceous marine green alga, Bryopsis Corticulans. Photosynth Res. 2014 Sep 12.
Wientjes et al (2013). LHCII is an antenna of both photosystems after long-term acclimation. BBA, Jan 6.
Rudowska et al. (2012). Chloroplast biogenesis - correlation between structure and function. BBA, available on line, March 2012.
Lan et al (2023) UPL5 modulates WHY2 protein distribution in a Kub-site dependent ubiquitination in response to [Ca2+]cyt-induced leaf senescence - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Oxygenic photosynthesis poster by prof. Govindjee and Dr. Shevela
Z-scheme of photosynthetic electron transport by prof. Govindjee and Dr. Björn and Dr. Shevela
- Reviews:
-
Soo Yeon Ko | 2020-11-18We always use this antibody when we check Lhcb3 band in Western blot(1:7000 dilution). It is working on Oriza Sativa.Soo Yeon Ko | 2019-11-18The antibody is working on Oriza Sativa. We used isolated 2ug thylakoid membrane and can get a band (1:5000 dilution) clearlyK. Browning | 2015-07-21Works well with Arabidopsis total and chloroplast extracts. Some cross reactivity in total extracts with bands ~38 and ~75 kDa with overnight incubation in cold, but no cross reactive bands for chloroplast extracts when incubated only 1-2 hr at RT.Maciej Garstka | 2009-03-19specific to pea, bean and rye


