1
Anti-Lhca4 | PSI type IV chlorophyll a/b-binding protein
AS01 008 | Clonality: Polyclonal | Host: Rabbit | Reactivity: Monocots and dicots including A.thaliana, C. reticulata, F. margarita Swingle, H.vulgare, Nicotiana tabacum, O. sativa, P. patens, S. lycopersicum, S.oleracea, T. aestivum, Triticale, Z.mays
- Product Info
-
Immunogen: BSA-conjugated synthetic peptide derived from the Lhca4 protein ofArabidopsis thaliana UniProt: P27521, TAIR: At3g47470. This sequence is highly conserved in Lhca4 proteins of angiosperms (monocots and dicots) and gymnosperms.
Host: Rabbit Clonality: Polyclonal Purity: Total IgG. Protein G purified in PBS pH 7.4. Format: Lyophilized Quantity: 0.5 mg Reconstitution: For reconstitution add 100 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Western blot (WB) Recommended dilution: 1 : 2000-1 : 5000 (WB) Expected | apparent MW: 27.7 | 21 kDa for Arabidopsis thaliana
- Reactivity
-
Confirmed reactivity: Arabidopsis thaliana, Bryopsis corticulans, Citrus reticulata, Echinochloa crus-galli, Fortunella margarita Swingle, Hordeum vulgare, Mesembryanthemum crystallinum, Nicotiana tabacum, Oryza sativa, Physcomitrium patens, Pisum sativum, Posidonia oceanica, Spinacia oleracea, Triticum aestivum, Triticale, Zea mays Predicted reactivity: Dicots, Gymnosperms Not reactive in: No confirmed exceptions from predicted reactivity are currently known - Application Examples
-
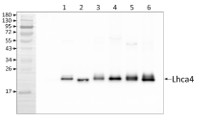
Application example 
1 µg of chlorophyll from Pisum sativum (1), Mesembryanthemum crystallinum (2), mesophyll (3) and bundle sheath (4) of Zea mays, mesophyll (5) and bundle sheath (6) of Echinochloa crus-galli chloroplasts extracted with 0.4 M sorbitol, 50 mM Hepes NaOH, pH 7.8, 10 mM NaCl, 5 mM MgCl2 and 2 mM EDTA were loaded to lanes. Samples were denatured with Laemmli buffer at 75 0C for 5 min and were separated on 12% SDS-PAGE, and blotted 30 min to PVDF using wet transfer. Blot was blocked with 5% milk for 2h at room temperature (RT) with agitation. Blot was incubated in the primary antibody Anti-Lhca4 (LOT 1908) at a dilution of 1: 3000 in 1% milk in TBS-T overnight at 4°C with agitation. The antibody solution was decanted and the blot was washed 4 times for 5 min in TBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG HRP conjugated, from Agrisera, AS09 602, LOT 1905) diluted to 1:20 000 in 1 % milk in TBS-T for 1h at RT with agitation. The blot was washed 5 times for 5 min in TBS-T and 2 times for 5 min in TBS, and developed for 1 min with 1.25 mM luminol, 0.198 mM coumaric acid and 0.009% H2O2 in 0.1 M Tris- HCl, pH 8.5. Exposure time in ChemiDoc System was 30 seconds.
Courtesy of Dr. Wioleta Wasilewska-Dębowska, Warsaw University, Poland
Application examples: 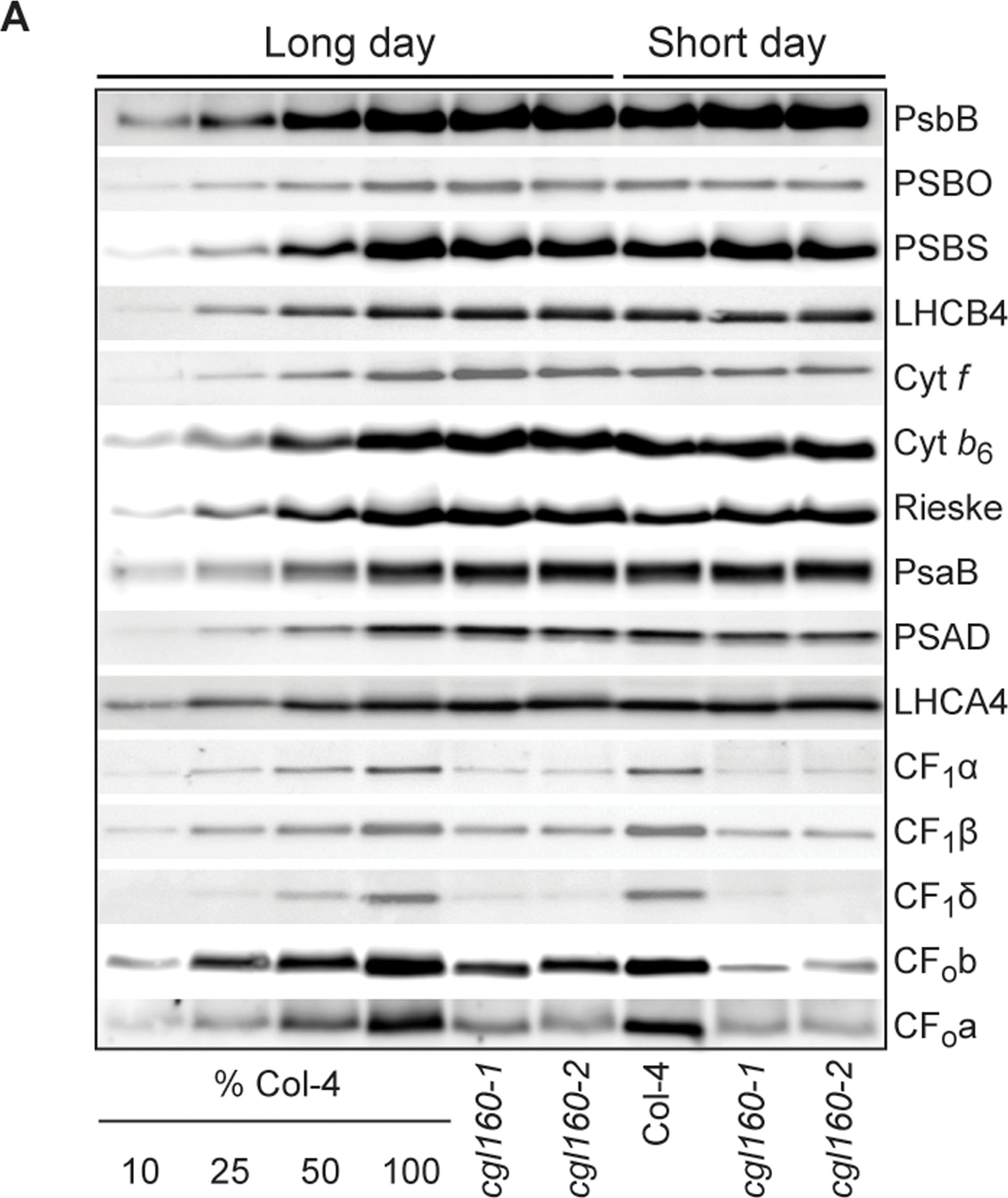
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 25835989
Journal: PLoS One
Figure Number: 7A
Published Date: 2015-04-04
First Author: Fristedt, R., Martins, N. F., et al.
Impact Factor: 2.942
Open PublicationAltered protein accumulation and stability of the chloroplast ATP synthase in the cgl160 mutant visualized by immunoblotting.A. Immunoblots with antibodies against essential subunits of the photosynthetic protein complexes of wild-type (Col-4) Arabidopsis and the two cgl160 T-DNA insertion lines grown under long-day and short-day conditions. Isolated thylakoid membranes were used, and equal amounts of chlorophyll were loaded onto the SDS-PAGE gel. For approximate quantification, wild-type samples from long-day plants were diluted to 10%, 25% and 50%, respectively. Accumulation of PSII was probed with antibodies against PsbB and PSBO. Additionally, the PSBS protein involved in NPQ and the minor PSII antenna protein LHCB4 were probed. Accumulation of the cytochrome b6f complex was probed with antibodies against the essential subunits PetA (cytochrome f), PetB (cytochrome b6), and PETC (Rieske protein). Accumulation of PSI was probed with antibodies against the reaction center subunit PsaB and the stromal ridge subunit PsaD. ATP synthase accumulation was probed with antibodies against the CF1 subunits AtpA (CF1?), AtpB (CF1?) and AtpD (CF1?) and antibodies against the CF0 subunits AtpF (CF0b) and AtpI (CF0a). B. Loading difference estimation for immunoblotting CF1 between wild type and cgl160-1. To obtain similar immunoblotting signal three times more (15 ?g protein) was needed for cgl160-1 compared to wild type (5 ?g protein). C. Maintenance of CF1 was measured by incubating leaves from wild type and cgl160-1 in solution containing the plastid protein synthesis inhibitor chloramphenicol for the indicated time points. Protein extract was isolated and separated by SDS-PAGE, immunoblotted and probed with specific antibodies against CF1 and LHCB2.1. Three times more protein was loaded from the mutant to obtain equal level of CF1 immunoblotting signal, as specified in B.
Reactant: Nicotiana tabacum (Common tobacco)
Application: Western Blotting
Pudmed ID: 28180288
Journal: J Exp Bot
Figure Number: 5A
Published Date: 2017-02-01
First Author: Schöttler, M. A., Thiele, W., et al.
Impact Factor: 6.088
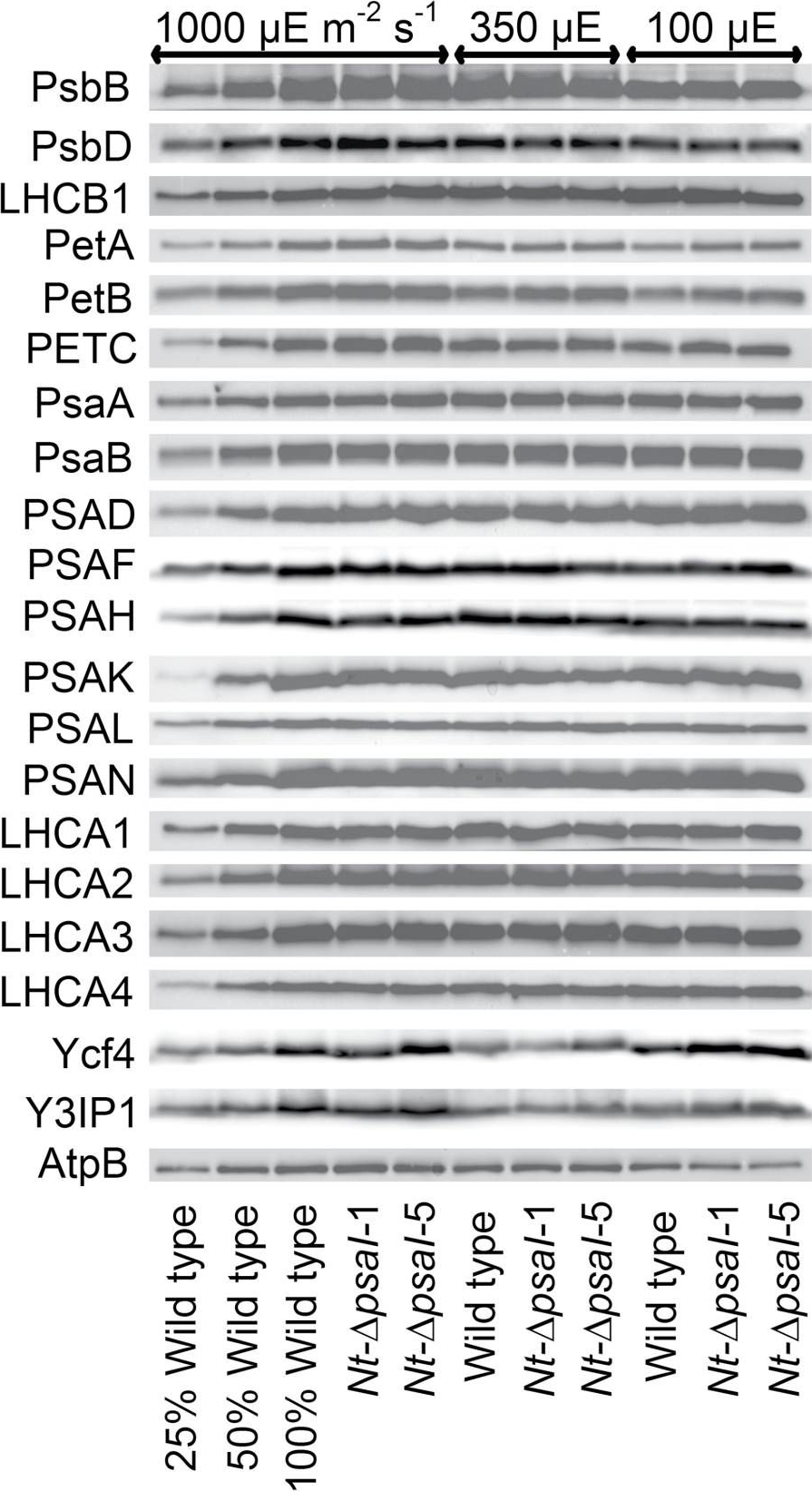
Open PublicationImmunoblot analysis of photosynthetic complex accumulation in wild-type tobacco and the two ?psaI lines grown under low, intermediate, and high-light conditions. Because the accumulation of most tested proteins was highest under high-light conditions, lanes one to three contain samples diluted to 25%, 50%, and a 100% sample of wild-type tobacco grown under high-light conditions, to allow for semi-quantitative determination of changes in protein abundance. Lanes four and five contain the two transplastomic lines grown at 1000 µE m?2 s?1. Lanes six to eight contain wild-type tobacco and the mutants grown at intermediate light intensities, and lanes nine to eleven contain samples grown at low light intensities. For PSII, the accumulation of the essential subunits PsbB (CP43) and PsbD (D2) and the LHCB1 antenna protein were determined, while for the cytochrome b6f complex, the accumulation of the essential redox-active subunits PetA (cytochrome f), PetB (cytochrome b6), and PETC (Rieske FeS protein) was tested. AtpB was probed as an essential subunit of the chloroplast ATP. For PSI, in addition to the three essential plastome-encoded subunits PsaA, PsaB, and PsaC, the accumulation of the nuclear-encoded subunits PSAD, PSAH, PSAK, PSAL, and PSAN and of the four LHCI proteins (LHCA1, LHCA2, LHCA3, LHCA4) was determined. Finally, we examined the accumulation of Ycf4, the chloroplast-encoded PSI-biogenesis factor encoded in the same operon as PsaI, and the nuclear-encoded assembly factor Y3IP1.
Reactant: Plant
Application: Western Blotting
Pudmed ID: 29423236
Journal: Hortic Res
Figure Number: 3A
Published Date: 2018-02-10
First Author: Zhu, M., Lin, J., et al.
Impact Factor: 6.072
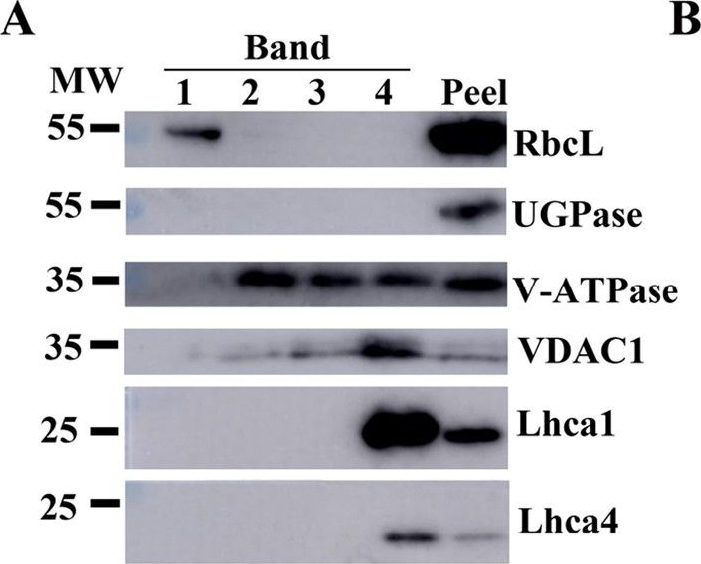
Open PublicationAssessment of the purity of isolated elaioplasts using immunoblots.Different fractions of the sucrose gradient (Band 1 to Band 4) are compared to peel proteins using antibodies for plastid stroma large Rubisco subunit (RbcL), cytosolic UGPase, vacuolar (v)-ATPase, mitochondrial voltage-dependent anion-selective channel protein 1 (VDAC1), and photosynthesis Light-harvesting complex (Lhca1 and Lhca4); b Coomassie blue protein profiles of purified elaioplasts (Band 1 to Band 4) from kumquat peel, as compared with purified chromoplast (Chro.) from sweet orange flesh and total kumquat peel proteins
- Additional Information
-
Additional information (application): Protein is processed into mature form (Jansson 1999). - Background
-
Background: The light-harvesting protein Lhca4 is one of the four main and highly conserved types of chlorophyll a/b-binding proteins (Lhca1-4) of the light harvesting antenna (LHCI) of plant photosystem I. Lhca4 is imported as a precursor from the cytosol into the chloroplast. Upon insertion into the thylakoid membrane Lhca4 forms a heterodimer (LHCI-730) with Lhca1 that associates with the PSI core close to PsaG and PsaF.
A biochemical characterization of the plant LHCI antenna can be found in Klimmek et al. (2005) The structure of the higher plant light harvesting complex I: in vivo characterization and structural interdependence of the Lhca proteins. Biochemistry 44: 3065–3073. - Product Citations
-
Selected references: Sarvari et al. (2022). Qualitative and quantitative evaluation of thylakoid complexes separated by Blue Native PAGE. Plant Methods. 2022 Mar 3;18(1):23. doi: 10.1186/s13007-022-00858-2. PMID: 35241118; PMCID: PMC8895881.
Ivanov et al. (2022) The decreased PG content of pgp1 inhibits PSI photochemistry and limits reaction center and light-harvesting polypeptide accumulation in response to cold acclimation. Planta 255, 36 (2022). https://doi.org/10.1007/s00425-022-03819-0
Zhu et al. (2020). A NAC transcription factor and its interaction protein hinder abscisic acid biosynthesis by synergistically repressing NCED5 in Citrus reticulata. J Exp Bot. 2020 Jun 22;71(12):3613-3625.doi: 10.1093/jxb/eraa118.
Forlani et al. (2020. HEBE, a novel positive regulator of senescence in Solanum lycopersicum. Sci Rep. 2020 Jul 3;10(1):11021.doi: 10.1038/s41598-020-67937-z.
Chen et al. (2019). Effects of Stripe Rust Infection on the Levels of Redox Balance and Photosynthetic Capacities in Wheat. Int J Mol Sci. 2019 Dec 31;21(1). pii: E268. doi: 10.3390/ijms21010268.
Mao et al. (2018). Comparison on Photosynthesis and Antioxidant Defense Systems in Wheat with Different Ploidy Levels and Octoploid Triticale. Int J Mol Sci. 2018 Oct 2;19(10). pii: E3006. doi: 10.3390/ijms19103006.
Li et al. (2018). Modulating plant growth-metabolism coordination for sustainable agriculture. Nature. 2018 Aug 15. doi: 10.1038/s41586-018-0415-5.
Zhu et al. (2018). A comprehensive proteomic analysis of elaioplasts from citrus fruits reveals insights into elaioplast biogenesis and function. Hortic Res. 2018 Feb 7;5:6. doi: 10.1038/s41438-017-0014-x.
Myouga et al. (2018). Stable accumulation of photosystem II requires ONE-HELIX PROTEIN1 (OHP1) of the light harvesting-like family. Plant Physiol. 2018 Feb 1. pii: pp.01782.2017. doi: 10.1104/pp.17.01782.
Schottler et al. (2017). The plastid-encoded PsaI subunit stabilizes photosystem I during leaf senescence in tobacco. J Exp Bot. 2017 Feb 1;68(5):1137-1155. doi: 10.1093/jxb/erx009.
Tyuereva et al. (2017). The absence of chlorophyll b affects lateral mobility of photosynthetic complexes and lipids in grana membranes of Arabidopsis and barley chlorina mutants. Photosynth Res. 2017 Apr 5. doi: 10.1007/s11120-017-0376-9. (Hordeum vulgare, western blot)
Yang-Er Chen et al. (2017). Responses of photosystem II and antioxidative systems to high light and high temperature co-stress in wheat. J. of Exp. Botany, Volume 135, March 2017, Pages 45–55.
Nath et al. (2016). A Nitrogen-Fixing Subunit Essential for Accumulating 4Fe-4S-Containing Photosystem I Core Proteins. Plant Physiol. 2016 Dec;172(4):2459-2470.
Fristedt et al. (2015). The thylakoid membrane protein CGL160 supports CF1CF0 ATP synthase accumulation in Arabidopsis thaliana. PLoS One. 2015 Apr 2;10(4):e0121658. doi: 10.1371/journal.pone.0121658.
Yokono et al. (2015). A megacomplex composed of both photosystem reaction centres in higher plants. Nat Commun. 2015 Mar 26;6:6675. doi: 10.1038/ncomms7675.
Qin et al. (2014). Isolation and characterization of a PSI-LHCI super-complex and its sub-complexes from a siphonaceous marine green alga, Bryopsis Corticulans. Photosynth Res. 2014 Sep 12. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Oxygenic photosynthesis poster by prof. Govindjee and Dr. Shevela
Z-scheme of photosynthetic electron transport by prof. Govindjee and Dr. Björn and Dr. Shevela - Reviews:
-
Soo Yeon Ko | 2019-11-18The antibody is also working on Oriza Sativa. We used isolated 2ug thylakoid membrane and can get the band (1:10000 dilution) clearlyMonica Colombo | 2015-01-30The antibody gives a good result at 1:2000 in Arabidopsis thaliana with 3ug of proteins| 2011-01-25The antibody worked very well on WB for the extracts from Arabidopsis cultured cells containing 160 ng of Chla at 1:10000 using ECL-Plus.| 2011-01-14Worked well for pumpkin at 1:2500 with 10 ug protien loaded.



