1
Anti-Lhca1 | PSI type I chlorophyll a/b-binding protein
AS01 005 | Clonality: Polyclonal | Host: Rabbit | Reactivity: Monocots and dicots including A. thaliana, A. hypogaea, C. reticulata, C. quitensis Kunt Bartl, E. crus-galli, F. margarita Swingle, G. hybrid, H. vulgare, L. esculentum (Solanum lycopersicum), N. tabacum, O. sativa, P. kurroa,, P. miliaceum, P. patens, P. sativum, P. strobus, P.vulgaris, S. lycopersicum, S. oleracea, Triticum aestivum, Z. mays
- Product Info
-
Immunogen: BSA-conjugated synthetic peptide derived from the Lhca1 protein of Arabidopsis thaliana UniProt: Q01667, TAIR: At3g54890. This sequence is highly conserved in Lhca1 proteins of angiosperms (monocots and dicots) and gymnosperms.
Host: Rabbit Clonality: Polyclonal Purity: Total IgG. Protein G purified in PBS pH 7.4. Format: Lyophilized Quantity: 0.5 mg Reconstitution: For reconstitution add 100 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Western blot (WB) Recommended dilution: 1 : 2000-1 : 5000 (WB) Expected | apparent MW: 25.99 | 22 kDa for Arabidopsis thaliana
- Reactivity
-
Confirmed reactivity: Arabidopsis thaliana, Arachis hypogaea, Bryopsis corticulans, Citrus retuculata, Colobanthus quitensis Kunt Bartl, Echinochloa crus-galli, Fortunella margarita Swingle, Guzmania hybrid, Hordeum vulgare, Lycopersicon esculentum (Solanum lycopersicum), Nicotiana tabacum, Oryza sativa, Panicum miliaceum, Picrorhiza kurroa, Physcomitrium patens, Pinus strobus, Pisum sativum, Phaseolus vulgaris, Posidonia oceanica, Solanum lycopresicum, Spinacia oleracea, Tillandsia flabellate, Triticale, Triticum aestivum, Zea mays Predicted reactivity: Dicots, Gymnosperms Not reactive in: No confirmed exceptions from predicted reactivity are currently known - Application Examples
-

Samples:
1 - MW marker
2 - 10 µg of Arabidopsis thaliana whole leaf extract
3 - 10 µg of Zea mays whole leaf extract
4- 10 µg of Solanum lycopersicum whole leaf extract10 µg/well of total protein extracted from , total cell extract, stored at -80ºC. Exact buffer components were: and denatured with Invitrogen LDS sample buffer (4X) at 70°C/5 min. Samples were separated on Invitrogen NuPage Bis-Tris 4-12% SDS-PAGE and blotted for 1 h to Invitrogen PVDF (pore size of 0.45 µm), using: wet transfer. Blot was blocked with 5% milk in TBS-T for: ON/4°C with agitation. Blot was incubated in the primary antibody at a dilution of 1: 1000 for 1h/RT with TBS-T Blocking. The antibody solution was decanted, and the blot was rinsed briefly twice, then washed once for 10 min and 2 times for 5 min in TBS-T at RT with agitation. Blot was incubated in matching secondary antibody (anti-rabbit IgG ALP conjugated, AS09 607 lot 2105 ) diluted to 1: 1 000 in TBS-T Blocking for 50 min/RT with agitation. The blot was washed as above and developed with Agrisera AS19 BCIP-NBT-PLUS lot 09269221 for 30 seconds. As soon as the desired band is detectable, briefly wash the membrane in generous amounts of deionized water. Transfer the membrane to fresh deionized water and incubate for 2 minutes with agitation. Change the water and incubate again for 5 minutes with agitation before placing the membrane on Whatman paper to dry. Image was captured after 1h.
Courtesy Agrisera
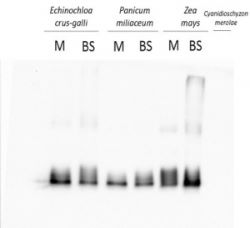
1.0 µg of chlorophyll from mesophyll (M) and bundle sheath (BS) thylakoids of various C4 plants (Echinochloa crus-galli, Panicum miliaceum, Zea mays) extracted with 0.4 M sorbitol, 50 mM Hepes NaOH, pH 7.8, 10 mM NaCl, 5 mM MgCl2 and 2 mM EDTA. Samples were denatured with Laemmli buffer at 75 0C for 5 min and were separated on 12% SDS-PAGE and blotted 30 min to PVDF using wet transfer. Blot was blocked with 5% milk for 1h at room temperature (RT) with agitation. Blot was incubated in the primary antibody at a dilution of 1: 2000 overnight at 4°C with agitation in 1% milk in TBS-T. The antibody solution was decanted and the blot was washed 4 times for 5 min in TBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, from Agrisera, AS09 602, Lot 1702) diluted to 1:25 000 in 1 % milk in TBS-T for 1h at RT with agitation. The blot was washed 5 times for 5 min in TBS-T and 2 times for 5 min in TBS, and developed for 1 min with 1.25 mM luminol, 0.198 mM coumaric acid and 0.009% H2O2 in 0.1 M Tris-HCl, pH 8.5. Exposure time in ChemiDoc System was 15 seconds.
Courtesy of Dr. Wioleta Wasilewska, Warsaw University, PolandApplication examples: 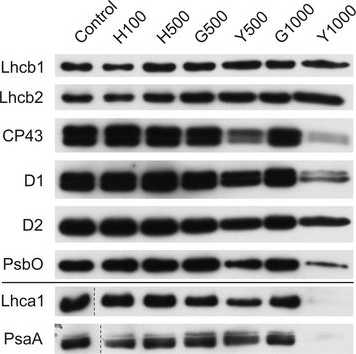
Reactant: Plant
Application: Western Blotting
Pudmed ID: 27590049
Journal: BMC Plant Biol
Figure Number: 9A
Published Date: 2016-09-02
First Author: Mazur, R., Sadowska, M., et al.
Impact Factor: 4.142
Open PublicationChanges of PSII and PSI antenna and core protein levels. Proteins from control and Tl-treated white mustard leaves were separated by SDS-PAGE followed by immunodetection with antibodies against Lhcb1, Lhcb2, Lhca1 (antenna proteins) and D1, D2, CP43, PsbO, PsaA (core proteins). Samples were loaded on the equal amount of chlorophyll (0.25 ?g). Description of samples abbreviation as given in the legend to Fig. 3
Reactant: Nicotiana tabacum (Common tobacco)
Application: Western Blotting
Pudmed ID: 28180288
Journal: J Exp Bot
Figure Number: 5A
Published Date: 2017-02-01
First Author: Schöttler, M. A., Thiele, W., et al.
Impact Factor: 6.088
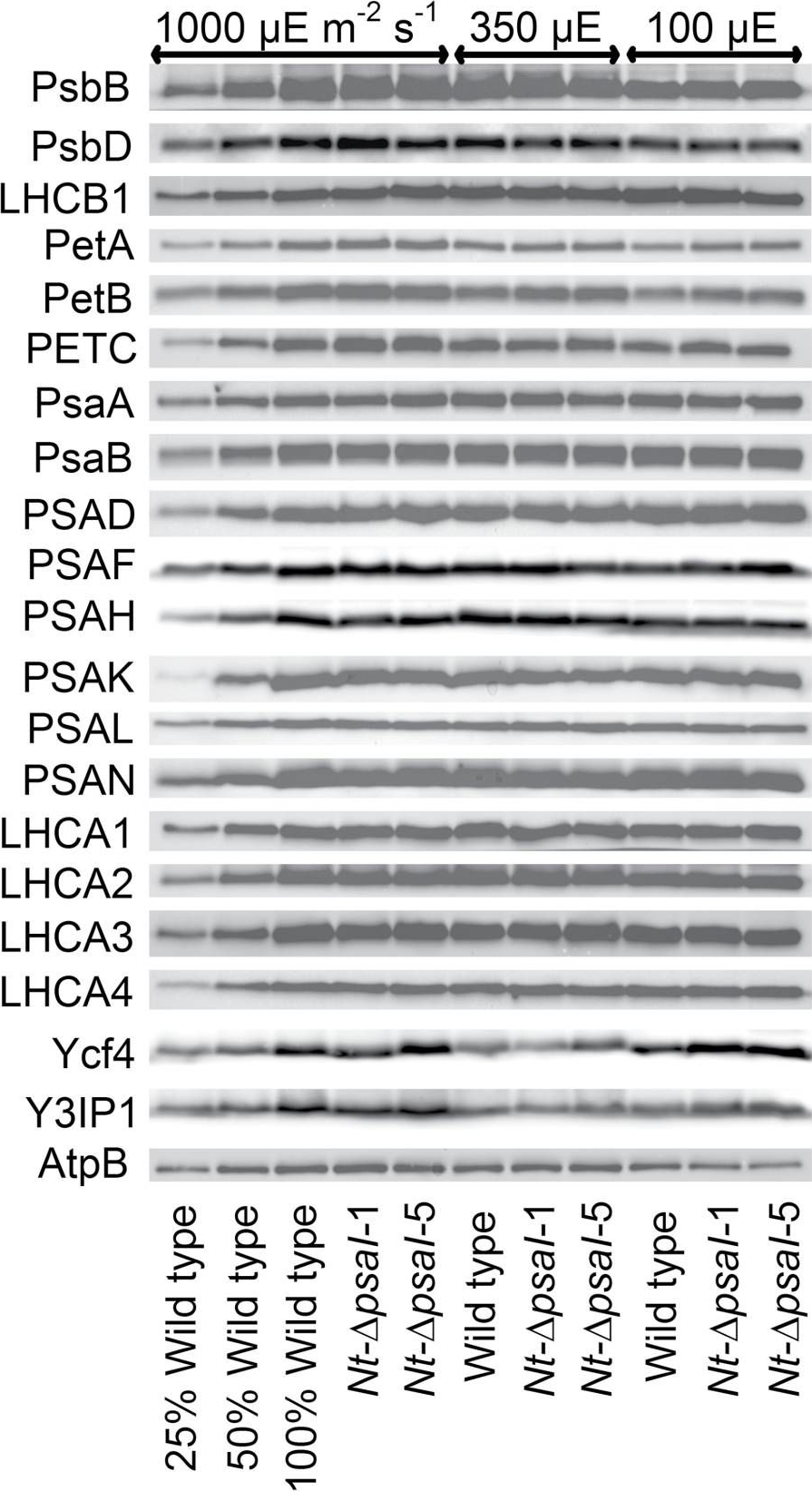
Open PublicationImmunoblot analysis of photosynthetic complex accumulation in wild-type tobacco and the two ?psaI lines grown under low, intermediate, and high-light conditions. Because the accumulation of most tested proteins was highest under high-light conditions, lanes one to three contain samples diluted to 25%, 50%, and a 100% sample of wild-type tobacco grown under high-light conditions, to allow for semi-quantitative determination of changes in protein abundance. Lanes four and five contain the two transplastomic lines grown at 1000 µE m?2 s?1. Lanes six to eight contain wild-type tobacco and the mutants grown at intermediate light intensities, and lanes nine to eleven contain samples grown at low light intensities. For PSII, the accumulation of the essential subunits PsbB (CP43) and PsbD (D2) and the LHCB1 antenna protein were determined, while for the cytochrome b6f complex, the accumulation of the essential redox-active subunits PetA (cytochrome f), PetB (cytochrome b6), and PETC (Rieske FeS protein) was tested. AtpB was probed as an essential subunit of the chloroplast ATP. For PSI, in addition to the three essential plastome-encoded subunits PsaA, PsaB, and PsaC, the accumulation of the nuclear-encoded subunits PSAD, PSAH, PSAK, PSAL, and PSAN and of the four LHCI proteins (LHCA1, LHCA2, LHCA3, LHCA4) was determined. Finally, we examined the accumulation of Ycf4, the chloroplast-encoded PSI-biogenesis factor encoded in the same operon as PsaI, and the nuclear-encoded assembly factor Y3IP1.
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 28430985
Journal: J Exp Bot
Figure Number: A
Published Date: 2017-04-01
First Author: Elena López-Calcagno, P., Omar Abuzaid, A., et al.
Impact Factor: 6.088
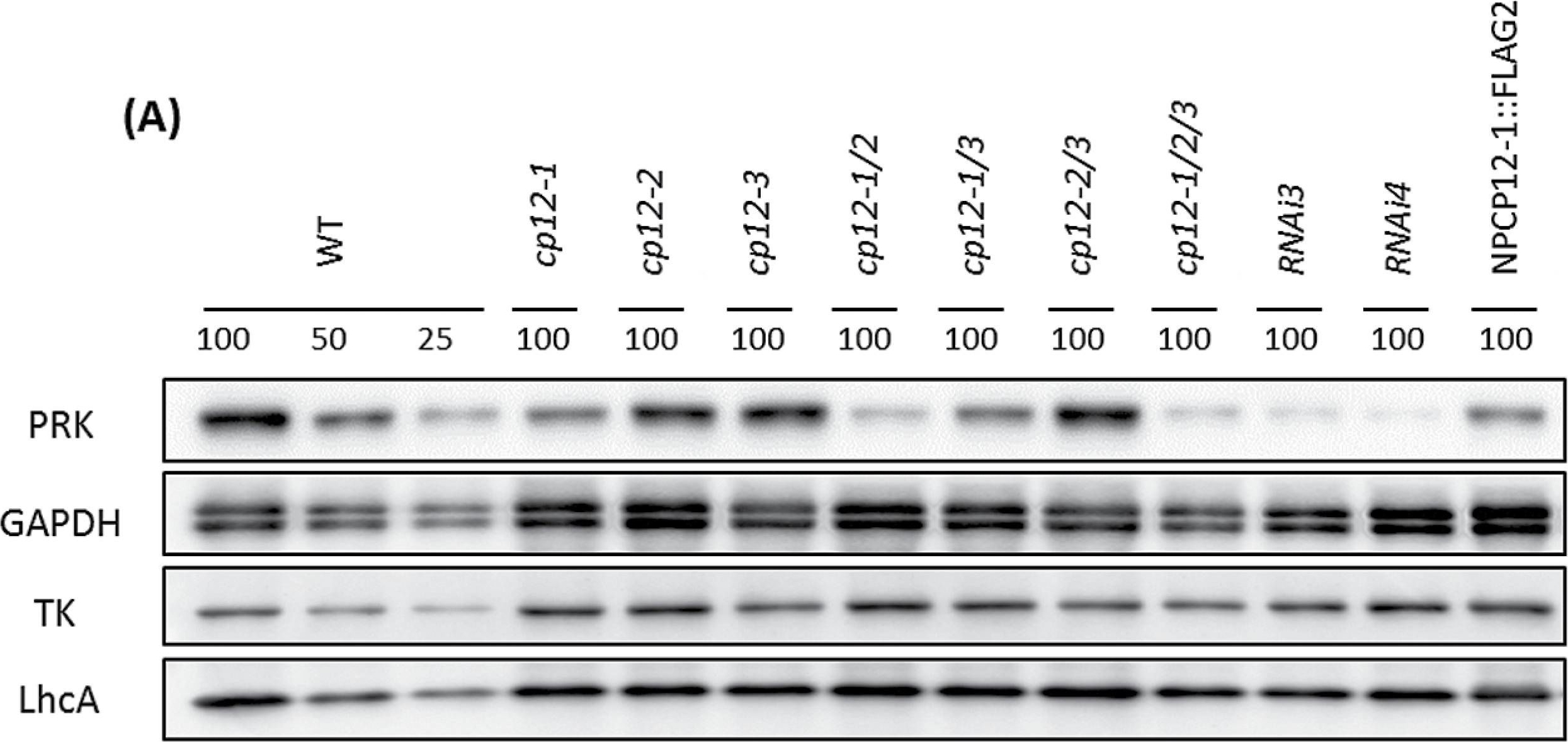
Open PublicationLack of CP12-1 leads to decreased levels of PRK protein. (A) Equal crude protein samples from mature rosettes of the indicated genotypes were analysed by immunoblotting using the antibodies shown. PRK (phosphoribulokinase), GAPDH (glyceraldehyde-3-phosphate dehydrogenase), TK (transketolase), and LhcA (light-harvesting complex). Each sample represents a pool of 2–4 different plants. (B) qRT-PCR analysis of PRK gene expression in CP12 mutants. Relative levels of expression of the PRK gene in the CP12-T-DNA mutant collection and cp12-1/2/3RNAi plants with respect to wild-type (WT) expression levels. The results are the mean from 3–4 biological replicates, and the error bars indicate the SE. Actin2 (AT3G18780), the elongation factor gene (AT1G07940.1), and Cyclophilin (AT2G29960) were used as internal standards (mean CV, 0.1869; mean M value, 0.4668).
Reactant: Plant
Application: Western Blotting
Pudmed ID: 29423236
Journal: Hortic Res
Figure Number: 3A
Published Date: 2018-02-10
First Author: Zhu, M., Lin, J., et al.
Impact Factor: 6.072
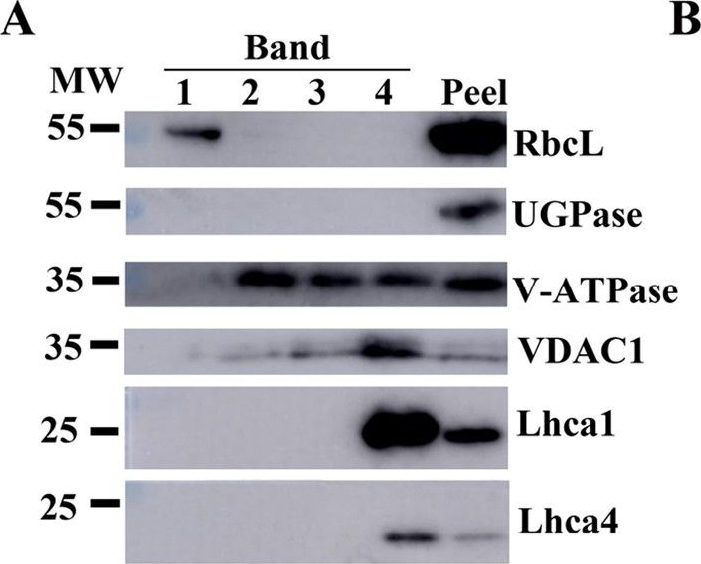
Open PublicationAssessment of the purity of isolated elaioplasts using immunoblots.Different fractions of the sucrose gradient (Band 1 to Band 4) are compared to peel proteins using antibodies for plastid stroma large Rubisco subunit (RbcL), cytosolic UGPase, vacuolar (v)-ATPase, mitochondrial voltage-dependent anion-selective channel protein 1 (VDAC1), and photosynthesis Light-harvesting complex (Lhca1 and Lhca4); b Coomassie blue protein profiles of purified elaioplasts (Band 1 to Band 4) from kumquat peel, as compared with purified chromoplast (Chro.) from sweet orange flesh and total kumquat peel proteins
Reactant: Chlamydomonas reinhardtii (Green Alga)
Application: Western Blotting
Pudmed ID: 29875782
Journal: Front Plant Sci
Figure Number: 5E
Published Date: 2018-06-08
First Author: Zhang, W., Zhong, H., et al.
Impact Factor: 5.435
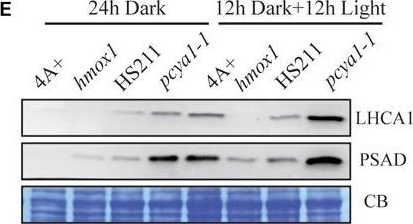
Open PublicationCharacterization of the CTE-disrupted pcya1-1 mutant. (A) Schematic diagram of an AphVIII insertion cassette into the PCYA1 locus in the pcya1-1 mutant. Black solid line represents 5?UTR and 3?UTR; black dotted line symbolizes intron; black box stands for exon and black arrows indicate the targeting sites of PCR primers. Purple, red, blue, and orange solid lines represent TP, NTE, FDBR and CTE regions of PCYA1, respectively. (B) PCR confirmation of the AphVIII insertion in pcya1-1. Genomic DNA was extracted from 4A+, HS211 and pcya1-1, respectively. PCR were performed using primers depicted in (A). HS211, the parental strain of pcya1-1. (C) Detection of CrPCYA1 expression in the dark or under light (?160 ?mol photons m-2s-1). Upper, immunoblot analysis of CrPCYA1 protein accumulation using antibody against CrPCYA1 mature protein; lower, Coomassie blue stain as an equal loading control. (D) Comparison of phototrophic growth phenotypes of pcya1-1 with 4A+, hmox1 and HS211 under constant low light (?60 ?mol photons m-2s-1), high light (?700 ?mol photons m-2s-1) and 12 h dark/12 h light cycle. (E) Accumulation of the photosystem I related proteins in pcya1-1, 4A+, HS211 and hmox1 in the dark or under light (?160 ?mol photons m-2s-1). Immunoblot analyses were performed with LHCA1 and PSAD antibodies. Coomassie blue stain (CB) as an equal loading control. Approximately 30 ?g total proteins were loaded in each lane.
Reactant: Chlamydomonas reinhardtii (Green Alga)
Application: Western Blotting
Pudmed ID: 29875782
Journal: Front Plant Sci
Figure Number: 6C
Published Date: 2018-06-08
First Author: Zhang, W., Zhong, H., et al.
Impact Factor: 5.435
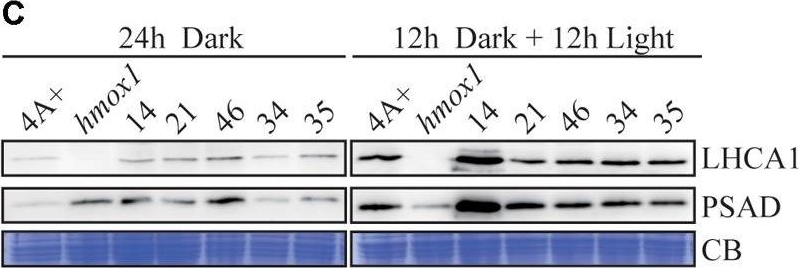
Open PublicationPCYA1 knockdown by artificial microRNA. (A) Analysis of CrPCYA1 protein accumulation in artificial microRNA-mediated PCYA1 knockdown lines. (B) Phototrophic growth of PCYA1 knockdown mutants were compared with 4A+ and hmox1 under constant low light, high light and dark/light cycle. Low light, ?60 ?mol photons m-2s-1; High light, ?700 ?mol photons m-2s-1. (C) Accumulation of representative photosystem I related proteins in PCYA1 knockdown mutants, 4A+ and hmox1 in the dark or under light (?160 ?mol photons m-2s-1). LHCA1 and PSAD antibodies were used for immunoblotting analyses. Around 30 ?g total proteins were loaded in each lane.
- Additional Information
-
Additional information: Antibody format is a total IgG fraction, which means that it is a pool of polyclonal antibodies obtained by purification of serum on Protein G, not on a specific antigen column.
This product can be sold containing ProClin if requested.Additional information (application): Protein is processed into mature form (Jansson 1999). - Background
-
Background: The light-harvesting protein Lhca1 is one of the four main and highly conserved types of chlorophyll a/b-binding proteins (Lhca1-4) of the light harvesting antenna (LHCI) of plant photosystem I. Lhca1 is imported as a precursor from the cytosol into the chloroplast. Upon insertion into the thylakoid membrane Lhca1 forms a heterodimer (LHCI-730) with Lhca4 that associates with the PSI core close to PsaG and PsaF.
A biochemical characterization of the plant LHCI antenna can be found in Klimmek et al. (2005) The structure of the higher plant light harvesting complex I: in vivo characterization and structural interdependence of the Lhca proteins. Biochemistry 44: 3065–3073 - Product Citations
-
Selected references: Collombat et al. (2025). Arabidopsis conditional photosynthesis mutants abc1k1 and var2 accumulate partially processed thylakoid preproteins and are defective in chloroplast biogenesis. Commun Biol . 2025 Jan 22;8(1):111. doi: 10.1038/s42003-025-07497-y.
Hang et al. (2024). HOT3/eIF5B1 confers Kozak motif-dependent translational control of photosynthesis-associated nuclear genes for chloroplast biogenesis. Nat Commun. 2024 Nov 14;15(1):9878. doi: 10.1038/s41467-024-54194-1.
Sakurabata et al. (2024). HASTY-mediated miRNA dynamics modulate nitrogen starvation-induced leaf senescence in Arabidopsis. Nat Commun. 2024 Sep 10;15(1):7913. doi: 10.1038/s41467-024-52339-w.
Frangedakis et al. (2024). MYB-related transcription factors control chloroplast biogenesis. Cell: DOI:https://doi.org/10.1016/j.cell.2024.06.039.
Hu et al. (2023). Drought affects both photosystems in Arabidopsis thaliana. New Phytol. 2023 Oct;240(2):663-675. doi: 10.1111/nph.19171. Epub 2023 Aug 2.
Wu et al (2023) Disruption of LEAF LESION MIMIC 4 affects ABA synthesis and ROS accumulation in rice.
Hu C, Mascoli V, Elias E, Croce R. The photosynthetic apparatus of the CAM plant Tillandsia flabellate and its response to water deficit. J Plant Physiol. 2023 Mar;282:153945. doi: 10.1016/j.jplph.2023.153945.
Jin et al. (2023) Dual roles for CND1 in maintenance of nuclear and chloroplast genome stability in plants. Cell Rep. 2023 Mar 28;42(3):112268. doi: 10.1016/j.celrep.2023.112268. Epub 2023 Mar 17.
Harchouni et al. (2022) Guanosine tetraphosphate (ppGpp) accumulation inhibits chloroplast gene expression and promotes super grana formation in the moss Physcomitrium (Physcomitrella) patens. New Phytol. 2022;236(1):86-98. doi:10.1111/nph.18320
Espinoza-Corral & Lundquist. (2022) The plastoglobule-localized protein AtABC1K6 is a Mn2+-dependent kinase necessary for timely transition to reproductive growth. J Biol Chem. 2022 Apr;298(4):101762. doi: 10.1016/j.jbc.2022.101762. Epub 2022 Feb 22. PMID: 35202657; PMCID: PMC8956952.
Sarvari et al. (2022). Qualitative and quantitative evaluation of thylakoid complexes separated by Blue Native PAGE. Plant Methods. 2022 Mar 3;18(1):23. doi: 10.1186/s13007-022-00858-2. PMID: 35241118; PMCID: PMC8895881.
Xiong et al. (2022) a chloroplast nucleoid protein of bacterial origin linking chloroplast transcriptional and translational machineries, is required for proper chloroplast gene expression in Arabidopsis thaliana. Nucleic Acids Res. 2022 Jun 23;50(12):6715-34. doi: 10.1093/nar/gkac501. Epub ahead of print. PMID: 35736138; PMCID: PMC9262611.
Kumari et al. (2021) In-depth assembly of organ and development dissected Picrorhiza kurroa proteome map using mass spectrometry. BMC Plant Biol. 2021 Dec 22;21(1):604. doi: 10.1186/s12870-021-03394-8. PMID: 34937558; PMCID: PMC8693493.
Wada et al. (2021) Identification of a Novel Mutation Exacerbated the PSI Photoinhibition in pgr5/pgrl1 Mutants; Caution for Overestimation of the Phenotypes in Arabidopsis pgr5-1 Mutant. Cells. 2021 Oct 26;10(11):2884. doi: 10.3390/cells10112884. PMID: 34831107; PMCID: PMC8616342.
Cecchin et al (2021) LPA2 protein is involved in photosystem II assembly in Chlamydomonas reinhardtii. Plant J. 2021 Jul 4. doi: 10.1111/tpj.15405. Epub ahead of print. PMID: 34218480.
Chen et al. (2021)Degradation of the photosystem II core complex is independent of chlorophyll degradation mediated by Stay-Green Mg2+ dechelatase in Arabidopsis,Plant Science,Volume 307,2021,110902,ISSN 0168-9452,https://doi.org/10.1016/j.plantsci.2021.110902.
Zhu et al. (2020). A NAC transcription factor and its interaction protein hinder abscisic acid biosynthesis by synergistically repressing NCED5 in Citrus reticulata. J Exp Bot. 2020 Jun 22;71(12):3613-3625.doi: 10.1093/jxb/eraa118.
Forlani et al. (2020. HEBE, a novel positive regulator of senescence in Solanum lycopersicum. Sci Rep. 2020 Jul 3;10(1):11021.doi: 10.1038/s41598-020-67937-z.
Wang et al. (2020). Post-translational coordination of chlorophyll biosynthesis and breakdown by BCMs maintains chlorophyll homeostasis during leaf development. Nat Commun. 2020; 11: 1254.
Chen et al. (2019). Effects of Stripe Rust Infection on the Levels of Redox Balance and Photosynthetic Capacities in Wheat. Int J Mol Sci. 2019 Dec 31;21(1). pii: E268. doi: 10.3390/ijms21010268.
Krupinska et al. (2019). The nucleoid-associated protein WHIRLY1 is required for the coordinate assembly of plastid and nucleus-encoded proteins during chloroplast development. Planta. 2019 Jan 11. doi: 10.1007/s00425-018-03085-z.
Mao et al. (2018). Comparison on Photosynthesis and Antioxidant Defense Systems in Wheat with Different Ploidy Levels and Octoploid Triticale. Int J Mol Sci. 2018 Oct 2;19(10). pii: E3006. doi: 10.3390/ijms19103006.
Yoshida et al. (2018). Thioredoxin-like2/2-Cys peroxiredoxin redox cascade supports oxidative thiol modulation in chloroplasts. Proc Natl Acad Sci U S A. 2018 Aug 13. pii: 201808284. doi: 10.1073/pnas.1808284115.
Li et al. (2018). Modulating plant growth-metabolism coordination for sustainable agriculture. Nature. 2018 Aug 15. doi: 10.1038/s41586-018-0415-5.
Zhu et al. (2018). A comprehensive proteomic analysis of elaioplasts from citrus fruits reveals insights into elaioplast biogenesis and function. Hortic Res. 2018 Feb 7;5:6. doi: 10.1038/s41438-017-0014-x.
Myouga et al. (2018). Stable accumulation of photosystem II requires ONE-HELIX PROTEIN1 (OHP1) of the light harvesting-like family. Plant Physiol. 2018 Feb 1. pii: pp.01782.2017. doi: 10.1104/pp.17.01782.
Schottler et al. (2017). The plastid-encoded PsaI subunit stabilizes photosystem I during leaf senescence in tobacco. J Exp Bot. 2017 Feb 1;68(5):1137-1155. doi: 10.1093/jxb/erx009.
Yang et al. (2017). Tetratricopeptide repeat protein Pyg7 is essential for photosystem I assembly by interacting with PsaC in Arabidopsis. Plant J. 2017 Jun 21. doi: 10.1111/tpj.13618.
Tyuereva et al. (2017). The absence of chlorophyll b affects lateral mobility of photosynthetic complexes and lipids in grana membranes of Arabidopsis and barley chlorina mutants. Photosynth Res. 2017 Apr 5. doi: 10.1007/s11120-017-0376-9. (Hordeum vulgare, western blot)
Mazur et al. (2016). Overlapping toxic effect of long term thallium exposure on white mustard (Sinapis alba L.) photosynthetic activity. BMC Plant Biol. 2016 Sep 2;16(1):191. doi: 10.1186/s12870-016-0883-4.
Heinnickel et al. (2016). Tetratricopeptide repeat protein protects photosystem I from oxidative disruption during assembly. Proc Natl Acad Sci U S A. 2016 Mar 8;113(10):2774-9. doi: 10.1073/pnas.1524040113
Fristedt et al. (2015). The thylakoid membrane protein CGL160 supports CF1CF0 ATP synthase accumulation in Arabidopsis thaliana. PLoS One. 2015 Apr 2;10(4):e0121658. doi: 10.1371/journal.pone.0121658.
Qin et al. (2014). Isolation and characterization of a PSI-LHCI super-complex and its sub-complexes from a siphonaceous marine green alga, Bryopsis Corticulans. Photosynth Res. 2014 Sep 12.
Saito et al. (2014). Fe deficiency induces phosphorylation and translocation of Lhcb1 in barley thylakoid membranes. FEBS Lett. 2014 May 8. pii: S0014-5793(14)00317-2. doi: 10.1016/j.febslet.2014.04.031. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Oxygenic photosynthesis poster by prof. Govindjee and Dr. Shevela
Z-scheme of photosynthetic electron transport by prof. Govindjee and Dr. Björn and Dr. Shevela - Reviews:
-
Katya Georgieva | 2019-06-24The antibody worked very well on Haberlea rhodopensis thylakoid proteins in dilution 1:2000, 3 ug chlorophyll per lane, using ECL for detection.Monica Colombo | 2015-01-30The antibody worked very well in Arabidopsis thaliana at 1:2000 with 3ug of proteinsJirong Huang | 2011-09-14This antibody is one of the best antibodies we have bought from Agrisera.| 2011-01-25The antibody worked very well on WB for the extracts from Arabidopsis cultured cells containing 160 ng of Chla at 1:10000 using ECL-Plus.| 2011-01-14Worked very well for pumpkin at 1:5000 with 10 ug protein loaded.Sarah Croonenborghs | 2009-05-28the antibody worked excellent on a Guzmania hybrid (Bromeliaceae) with both dilutions, 1 : 2000 and 1 : 5000.Claus Schwechhiemer | 2009-05-08Worked very nice on WB with 15µg of total proteins extracted from Arabidopsis thaliana.Maciej Garstka | 2009-03-19specific to pea, less to bean



