1
Anti-Cyt f | Cytochrome f protein (PetA) of thylakoid Cyt b6/f-complex (algal)
AS06 119 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. thaliana, C. reinhardtii, C. quitensis Kunt Bartl, C. pumilum, H. vulgare, N. gaditana, N. tabacum, P. patens, S. martensi, Synechocystis 6803 substrain PCC-M, U. prolifera
- Product Info
-
Immunogen: GST fusion to cytochrome f from Chlamydomonas reinhartii P23577
Host: Rabbit Clonality: Polyclonal Purity: Serum Format: Lyophilized Quantity: 50 µl Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Western blot (WB) Recommended dilution: 1 : 2000-1 : 50 000 (WB) Expected | apparent MW: 34 | 31-32 kDa
- Reactivity
-
Confirmed reactivity: Arabidopsis thaliana, Chlamydomonas reinhardtii, Chlorella vulgaris, Colobanthus quitensis Kunt Bartl, CRaterostigma pumilum, Hordeum vulgare, Nannochloropsis gaditana, Nicotiana tabacum, Nostoc sp. PCC7120, Physcomitrium patens, Selaginella martensi, Synechocystis sp. 6803 substrain PCC-M, Ulva prolifera Predicted reactivity: Algae, Citrus x limon, Zea mays
Species of your interest not listed? Contact us - Application Examples
-
Application example

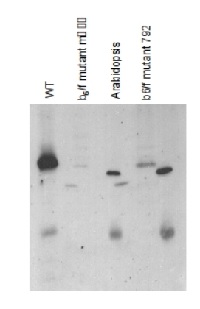
Chlamydomonas reinhardtii samples are lysed cells, Arabidopsis thaliana samples are prepared from thylakoid membranes; Samples were boiled 1 min in 3%SDS,then 10ug of chlorophylls were loaded in each well of a 12% acrylamide gel.

15 µg protein of whole cell extracts precipitated with 80 % acetone followed by re-suspension in 100 mM Tris HCl pH 6.8, 4 % SDS, 20 mM EDTA of Chlamydomonas reinhardtii were loaded on BioRad 4-20% Criterion TGX Precast Mini protein gels. Gels were run at 85V. For electro-blotting wet transfer onto nitrocellulose membrane was performed (105 V for 1h). Overnight blocking with 5% milk in TBS-T in the cold room. Two-hour-long incubation with anti-cyt f antibodies at a dilution of 1:50 000 in TBS-T was followed by washes and incubation with matching anti-rabbit secondary antibodies and development with chemiluminescent detection reagent following manufacture's recommendations. Image was taken using Chemidoc MP Imaging system. Exposure time was in a range of 3 to 10 seconds.
Courtesy of Dr. Angeliki Tsichla, Petroutsos lab, CEA Grenoble, FranceApplication examples: 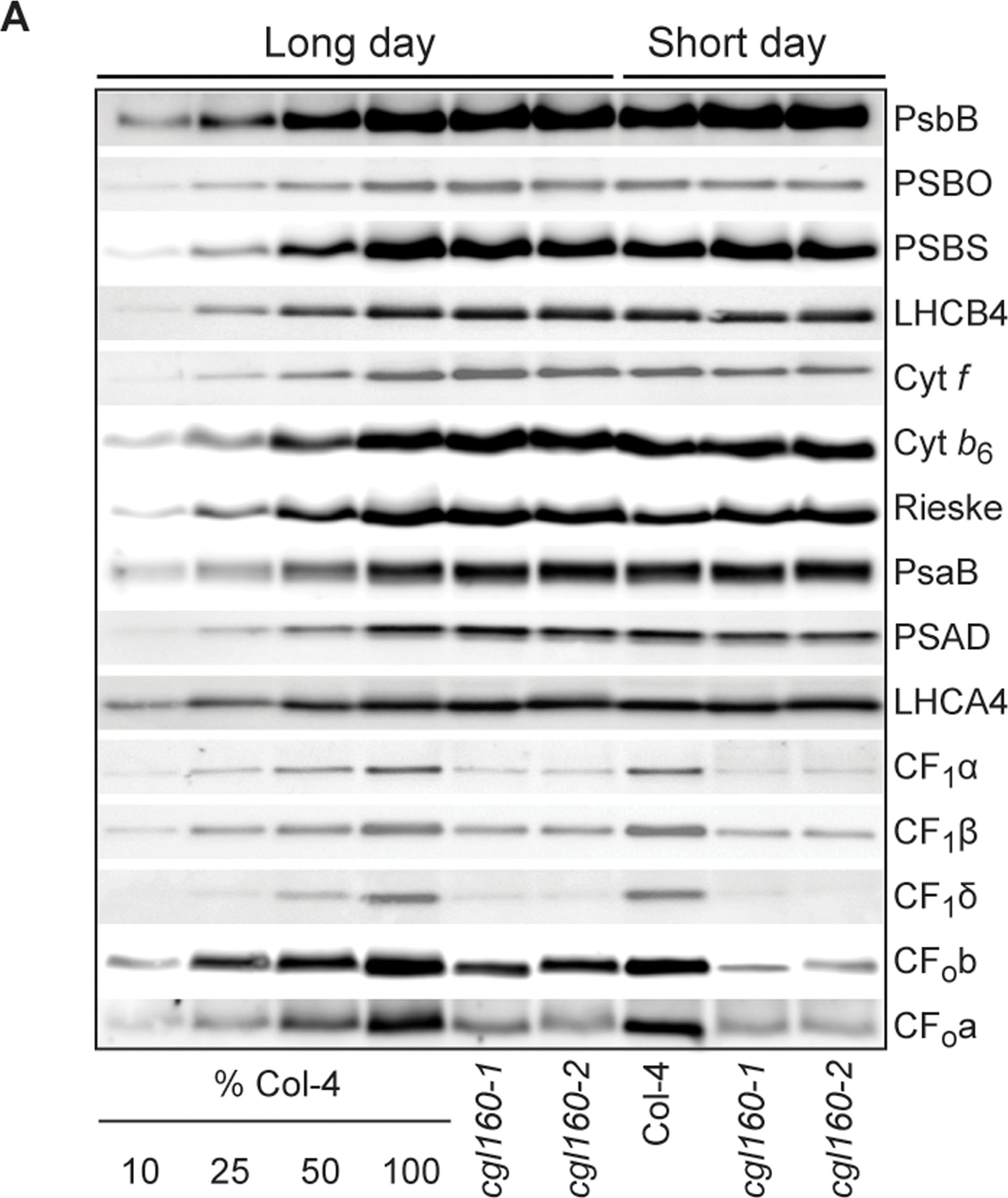
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 25835989
Journal: PLoS One
Figure Number: 7A
Published Date: 2015-04-04
First Author: Fristedt, R., Martins, N. F., et al.
Impact Factor: 2.942
Open PublicationAltered protein accumulation and stability of the chloroplast ATP synthase in the cgl160 mutant visualized by immunoblotting.A. Immunoblots with antibodies against essential subunits of the photosynthetic protein complexes of wild-type (Col-4) Arabidopsis and the two cgl160 T-DNA insertion lines grown under long-day and short-day conditions. Isolated thylakoid membranes were used, and equal amounts of chlorophyll were loaded onto the SDS-PAGE gel. For approximate quantification, wild-type samples from long-day plants were diluted to 10%, 25% and 50%, respectively. Accumulation of PSII was probed with antibodies against PsbB and PSBO. Additionally, the PSBS protein involved in NPQ and the minor PSII antenna protein LHCB4 were probed. Accumulation of the cytochrome b6f complex was probed with antibodies against the essential subunits PetA (cytochrome f), PetB (cytochrome b6), and PETC (Rieske protein). Accumulation of PSI was probed with antibodies against the reaction center subunit PsaB and the stromal ridge subunit PsaD. ATP synthase accumulation was probed with antibodies against the CF1 subunits AtpA (CF1?), AtpB (CF1?) and AtpD (CF1?) and antibodies against the CF0 subunits AtpF (CF0b) and AtpI (CF0a). B. Loading difference estimation for immunoblotting CF1 between wild type and cgl160-1. To obtain similar immunoblotting signal three times more (15 ?g protein) was needed for cgl160-1 compared to wild type (5 ?g protein). C. Maintenance of CF1 was measured by incubating leaves from wild type and cgl160-1 in solution containing the plastid protein synthesis inhibitor chloramphenicol for the indicated time points. Protein extract was isolated and separated by SDS-PAGE, immunoblotted and probed with specific antibodies against CF1 and LHCB2.1. Three times more protein was loaded from the mutant to obtain equal level of CF1 immunoblotting signal, as specified in B.
Reactant: Nicotiana tabacum (Common tobacco)
Application: Western Blotting
Pudmed ID: 28180288
Journal: J Exp Bot
Figure Number: 5A
Published Date: 2017-02-01
First Author: Schöttler, M. A., Thiele, W., et al.
Impact Factor: 6.088
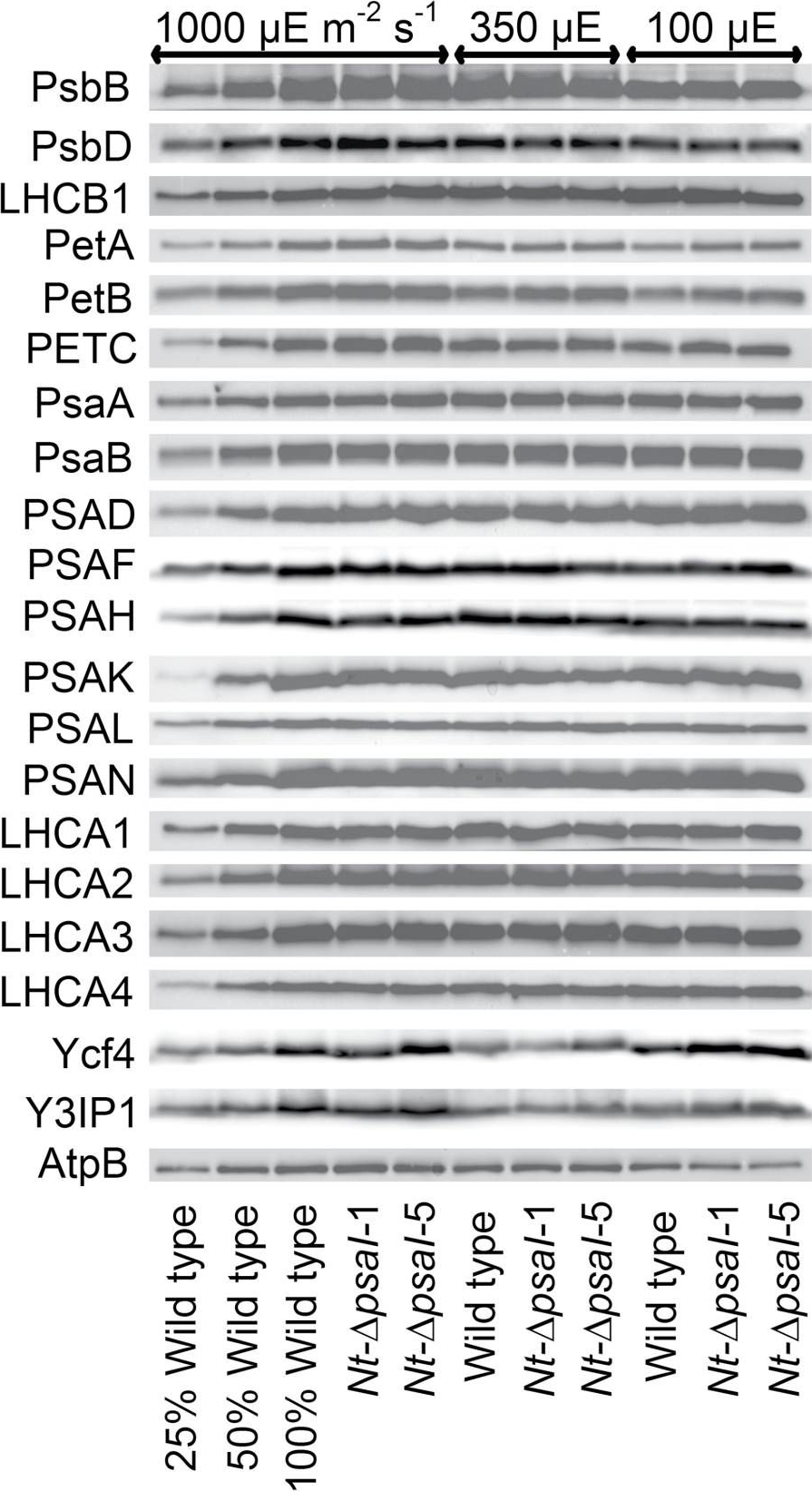
Open PublicationImmunoblot analysis of photosynthetic complex accumulation in wild-type tobacco and the two ?psaI lines grown under low, intermediate, and high-light conditions. Because the accumulation of most tested proteins was highest under high-light conditions, lanes one to three contain samples diluted to 25%, 50%, and a 100% sample of wild-type tobacco grown under high-light conditions, to allow for semi-quantitative determination of changes in protein abundance. Lanes four and five contain the two transplastomic lines grown at 1000 µE m?2 s?1. Lanes six to eight contain wild-type tobacco and the mutants grown at intermediate light intensities, and lanes nine to eleven contain samples grown at low light intensities. For PSII, the accumulation of the essential subunits PsbB (CP43) and PsbD (D2) and the LHCB1 antenna protein were determined, while for the cytochrome b6f complex, the accumulation of the essential redox-active subunits PetA (cytochrome f), PetB (cytochrome b6), and PETC (Rieske FeS protein) was tested. AtpB was probed as an essential subunit of the chloroplast ATP. For PSI, in addition to the three essential plastome-encoded subunits PsaA, PsaB, and PsaC, the accumulation of the nuclear-encoded subunits PSAD, PSAH, PSAK, PSAL, and PSAN and of the four LHCI proteins (LHCA1, LHCA2, LHCA3, LHCA4) was determined. Finally, we examined the accumulation of Ycf4, the chloroplast-encoded PSI-biogenesis factor encoded in the same operon as PsaI, and the nuclear-encoded assembly factor Y3IP1.
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 28791032
Journal: Front Plant Sci
Figure Number: 2A
Published Date: 2017-08-10
First Author: Kohzuma, K., Froehlich, J. E., et al.
Impact Factor: 5.435
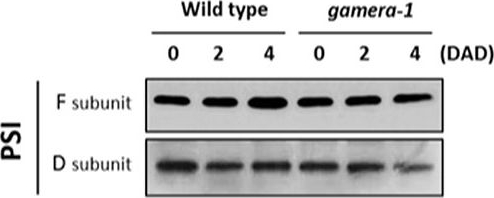
Open PublicationChanges in the protein levels of photosynthetic components under extended dark exposure in wild-type and gamera-1. Immunoblot detection of photosynthetic proteins from leaves of Ws and gamera-1 plants incubated after dark adaptation for 0, 2, and 4 days was examined. Specifically, essentially thylakoid fractions were assayed to determine the content of the following proteins: ?-subunit of ATP synthase; the D1 protein, OEC17, OEC23, and OEC33 of PSII; Cyt f and Rieske protein of the cytochrome b6f complex; and the F and D subunits of PSI, after extended dark treatment. Proteins were resolved via SDS-PAGE gel based on equal microgram chlorophyll per lane loading and processed as described in Section “Materials and Methods”. The Large subunit of RuBisco and LHCII stained with either CBB or Ponceau red, respectively, are presented here as loading controls. DAD indicates days after dark adaptation.
- Additional Information
-
Additional information: Contains 0.02 % sodium azide as preservative Additional information (application): Reaction with cyanobacteria: Synechocystis 6803 and Synechococcus 7942 possible to obtain on total cell extract when using antibody at 1: 500 and longer exposure time. - Background
-
Background: Multi-subunit complex of cytb6/f is a crucial component for the photosynthetic electron transport chain of higher plants, green algae and cyanobacteria. This complex is catalyzing oxidation of quinols and the reduction the reduction of plastocyanin. This reaction allows to establish the proton force required for the ATP synthesis. Four major subunits build the complex: the petA gene product corresponding to a c-type cytochrome (cytf), the petB gene product corresponding to a b-type/c’-type cytochrome with three haems (cyt b6), the petD gene product (subunit IV, or suIV), and the petC gene product, corresponding to the Rieske/Iron/sulfur protein.
- Product Citations
-
Selected references: Redekop et al. (2020). PsbS Contributes to Photoprotection in Chlamydomonas Reinhardtii Independently of Energy Dissipation . Biochim Biophys Acta Bioenerg . 2020 Jun 1;1861(5-6):148183.doi: 10.1016
Liu et al. (2020). Acid treatment combined with high light leads to increased removal efficiency of Ulva prolifera. Algal Research,Volume 45, January 2020, 101745
Storti et al. (2020). The activity of chloroplast NADH dehydrogenase-like complex influences the photosynthetic activity of the moss Physcomitrella patens. doi.org/10.1101/2020.01.29.924597
Koh et al. (2019). Heterologous synthesis of chlorophyll� b� in� Nannochloropsis salina� enhances growth and lipid production by increasing photosynthetic efficiency. Biotechnol Biofuels.� 2019 May 14;12:122. doi: 10.1186/s13068-019-1462-3. eCollection 2019.
Dall'Osto et al. (2019). Combined resistance to oxidative stress and reduced antenna size enhance light-to-biomass conversion efficiency in Chlorella vulgaris cultures.Biotechnol Biofuels. 2019 Sep 16;12:221. doi: 10.1186/s13068-019-1566-9.
Fristedt et al. (2015). The thylakoid membrane protein CGL160 supports CF1CF0 ATP synthase accumulation in Arabidopsis thaliana. PLoS One. 2015 Apr 2;10(4):e0121658. doi: 10.1371/journal.pone.0121658.
Storti et al. (2018). Role of cyclic and pseudo-cyclic electron transport in response to dynamic light changes in Physcomitrella patens. Plant Cell Environ. 2018 Nov 29. doi: 10.1111/pce.13493.
Kong et al. (2018) Interorganelle Communication: Peroxisomal MALATE DEHYDROGENASE2 Connects Lipid Catabolism to Photosynthesis through Redox Coupling in Chlamydomonas. Plant Cell. 2018 Aug;30(8):1824-1847. doi: 10.1105/tpc.18.00361
Jokel et al. (2018). Hunting the main player enabling Chlamydomonas reinhardtii growth under fluctuating light. Plant J. 2018 Mar 25. doi: 10.1111/tpj.13897.
Du et al. (2018). Galactoglycerolipid Lipase PGD1 Is Involved in Thylakoid Membrane Remodeling in Response to Adverse Environmental Conditions in Chlamydomonas. Plant Cell. 2018 Feb;30(2):447-465. doi: 10.1105/tpc.17.00446.
Schöttler et al. (2017). The plastid-encoded PsaI subunit stabilizes photosystem I during leaf senescence in tobacco. J Exp Bot.Ã? 2017 Feb 1;68(5):1137-1155. doi: 10.1093/jxb/erx009.
Zou et al. (2017). An Animal-Like Cryptochrome Controls the Chlamydomonas Sexual Cycle. Plant Physiol. 2017 Jul;174(3):1334-1347. doi: 10.1104/pp.17.00493.
Georg et al. (2017). Acclimation of Oxygenic Photosynthesis to Iron Starvation Is Controlled by the sRNA IsaR1. Curr Biol. 2017 May 22;27(10):1425-1436.e7. doi: 10.1016/j.cub.2017.04.010. (Synechocystis PCC6803)
Tyuereva et al. (2017). The absence of chlorophyll b affects lateral mobility of photosynthetic complexes and lipids in grana membranes of Arabidopsis and barley chlorina mutants. Photosynth Res. 2017 Apr 5. doi: 10.1007/s11120-017-0376-9. (Hordeum vulgare, western blot)
Ferroni et al. (2016). Light acclimation in the lycophyte Selaginella martensii depends on changes in the amount of photosystems and on the flexibility of the light-harvesting complex II antenna association with both photosystems. New Phytol. 2016 Apr 5. doi: 10.1111/nph.13939.
Suorsa et al. (2015). Light acclimation involves dynamic re-organisation of the pigment-protein megacomplexes in non-appressed thylakoid domains. Plant J. 2015 Aug 29. doi: 10.1111/tpj.13004.
Charuvi et al. (2015). Photoprotection Conferred by Changes in Photosynthetic Protein Levels and Organization during Dehydration of a Homoiochlorophyllous Resurrection Plant. Plant Physiol. 2015 Apr;167(4):1554-65. doi: 10.1104/pp.114.255794.
Hojka et al. (2014). Inducible repression of nuclear-encoded subunits of the cytochrome b6f complex in tobacco reveals an extraordinarily long lifetime of the complex. Plant Physiol. 2014 Jun 24. pii: pp.114.243741.
Dang et al. (2014). Combined Increases in Mitochondrial Cooperation and Oxygen Photoreduction Compensate for Deficiency in Cyclic Electron Flow in Chlamydomonas reinhardtii. Plant Cell. 2014 Jul 2. pii: tpc.114.126375.
Lang et al. (2011).Simultaneous isolation of pure and intact chloroplasts and mitochondria from moss as the basis for sub-cellular proteomics. Plant Cell Rep. 2011 Feb;30(2):205-15.doi: 10.1007/s00299-010-0935-4. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts - Reviews:
-
Saskia Zeilfelder | 2024-07-19We analyzed whole cell samples of Chlamydomonas reinhardtii using 10 µg total protein or 1 µg total chlorophyll per lane. After WB transfer we used an AB dilution of 1:10.000. The product worked really well and we obtained signals at approximately 32 kDa.Marie Bertrand | 2023-07-26We used this antibody on chlamydomanas protein extract.
With a 1:1000 dilution we get a verry strong signal. I recommand it.Benjamin Spaniol | 2021-02-18We used this antibody for Chlamydomonas reinhardtii whole cell protein extracts. After separating ~5 µg proteins via SDS-PAGE and western blotting, a dilution of 1:10.000 of the antibody resulted in a clear signal without any additional bands. Definitely recommended!Brendan | 2015-07-15Antibody works great with both Arabidopsis and Chlamydomonas total protein samples using a 1:10,000 dilution. Results are consistently without high background noise or extra bands.| 2014-12-204 µg of total protein from Phaeodactylum tricornutum were loaded on a 13% SDS-PAGE and blotted 1.5 h to nitrocellulose. Blots were blocked immediately following transfer in 5% milk-TBS-T for 1h at room temperature with agitation. Blots were incubated in the primary antibody at a dilution of 1:5000 overnight at 4 oC with agitation. The antibody solution was decanted and the blot was rinsed briefly, then washed 3 times for 5 min in TBS-T at room temperature with agitation. Blots were incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated) diluted to 1:10 000 in TBS-T for 1h at room temperature with agitation. The blots were washed as above and developed 60 sec with ECL detection reagent. Images of the blots were obtained using a CCD imager (Chemidock MP Imaging, Bio-Rad).


