1
Anti-Clathrin heavy-chain 1,2
AS10 690 | Clonality: Polyclonal | Host: Rabbit | Reactivity: Arabidopis thaliana, Chlamydomonas reinhardtii, Nicotiana tabacum
- Product Info
-
Immunogen: KLH-conjugated peptide derived from available plant clathrin heavy chain sequences including Arabidopsis thaliana clathrin heavy chain 1 UniProt: Q0WNJ6, TAIR:At3g11130, clathrin heavy chain 2 UniProt: Q0WLB5,TAIR:At3g08530
Host: Rabbit Clonality: Polyclonal Purity: Immunogen affinity purified serum in PBS pH 7.4. Format: Lyophilized Quantity: 50 µg Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Immunolocalization (IL), Immunofluorescence (IF), Western blot (WB) Recommended dilution: 1 : 2400 (IL), 1 :400 (IF), 1 : 2000 (WB) Expected | apparent MW: 193 | 170 kDa (Arabidopsis thaliana)
- Reactivity
-
Confirmed reactivity: Actinidia chinensis, Arabidopsis thaliana, Chlamydomonas reinhardtii, Nicotiana tabacumAS07 260 Predicted reactivity: Amborella trichopoda, Brassica napus, Capsella rubella, Citrus aurantium var. sinensis, Eucalyptus grandis, Glycine max, Chlorella variabilis, Leucaena glauca, Lotus japonicus, Medicago tribuloides, Mimulus guttatus, Musa malaccensis, Oryza sativa, Panicum italicum, Physcomitrium patens, Phaseolus vulgaris, Pisum sativum, Populus balsamifera, Populus trichocarpa, Ricinus communis, Selaginella moellendorffii, Sisymbrium salsugineum, Solanum lycopersicum, Theobroma cacao, Triticum aestivum, Vitis vinifera, Zea mays.
Species of your interest not listed? Contact usNot reactive in: Nicotiana benthamiana
- Application Examples
-
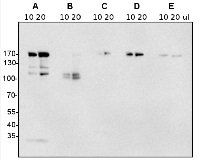
Application example 
10 or 20 µl of freshly prepared total protein from Arabidopsis thaliana suspension culture (A), 10 or 20 µl of total protein (older extract) from Arabidopsis thaliana suspension culture (B), 10 or 20 µl of total leaf extract from Nicotiana tabacum (C), 10 or 20 µl of freshly prepared total protein from Nicotiana tabacum leaf protoplasts (D), 10 or 20 µl total protein from older extract of Nicotiana tabacum leaf protoplasts (E) , extracted with buffer containing 100 mM Tris (pH 7.8), 200 mM NaCl, 1 mM EDTA, 2% (v/v) beta-mercaptoethanol and 0.2% (v/v) Triton X-100, were separated on 8% SDS-PAGE and blotted 2h to nitrocellulose (semidry blot at 200 mA and RT). Blots were blocked with 5% (w/v) milk powder and 1% (w/v) BSA over night at 4°C with agitation. Blot was incubated in the primary antibody at a dilution of 1:2 000 for 1h at RT with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG HRP conjugated) diluted to 1:10 000 in blocking solution for 45 min. at RT with agitation. The blot was washed as above and developed for 5 min with ECL according to the manufacturers instructions. Exposure time was 120 seconds.
Courtesy Fabian Künzl, University of Tuebingen, Germany
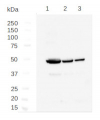
~10 µg (lane 1) and ~50 µg (lane 2) of Chlamydomonas reinhardtii whole-cell lysates were resolved by SDS-PAGE and transferred to PVDF membrane. Anti-clathrin heavy chain antibody (Agrisera AS10 690) was diluted 1:2500 in 5% (w/v) milk + 1% (w/v) fish skin gelatin in TBST and incubated overnight at 4° C. After washing and incubating with HRP-conjugated secondary antibody, diluted 1:10,000 for 30 minutes at room temperature, the membrane was washed and developed with chemiluminescent substrate of extreme low femtogram range, for 5 minutes following the manufacturer’s instructions. Exposure time on film was 5 seconds.
Courtesy of Dr. Branch Craige, University of Massachusetts Medical School, USA
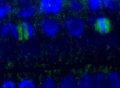
Cells were fixed with 4% paraformaldehyde, permeabilized, blocked in 3% fish skin gelatin +1% BSA in PBST, and incubated with anti-clathrin heavy chain antibody (diluted 1:2500, Agrisera AS10 690) and anti-acetylated tubulin (diluted 1:2000, Sigma T6793) antibody diluted in block overnight at 4° C. After washing and labeling with fluorescent secondary antibodies (Molecular Probes; clathrin: Alexa 488, green in the image; acetylated tubulin: Alexa 568, red in the image), the coverslips were mounted on Prolong Gold Anti-Fade (Molecular Probes) and imaged by widefield epifluorescence. Scale bar = 5 µm.
Courtesy of Dr. Branch Craige, University of Massachusetts Medical School, USA - Additional Information
-
Additional information (application): Please, note that fresh samples will provide better restuls (see image below) - Background
-
Background: Clathrin is a protein involved in intracellular trafficking and plays a major role in the formation of coated vesicles. It consists of three clathrin heavy chains and three light chains. Clathrin-coated vesicles (CCV) selectively sort cargo at the cell membrane, trans-Golgi network, and endosomal compartments for multiple membrane traffic pathways.
- Product Citations
-
Selected references: Takáč et al. (2024). Actin cytoskeleton and plasma membrane aquaporins are involved in different drought response of Arabidopsis rhd2 and der1 root hair mutants. Plant Physiology and Biochemistry Available online 19 September 2024, 109137.
Suanno et al. (2023) Small extracellular vesicles released from germinated kiwi pollen (pollensomes) present characteristics similar to mammalian exosomes and carry a plant homolog of ALIX, Front. Plant Sci., 25 January 2023, Sec. Plant Membrane Traffic and Transport, Volume 14 - 2023, https://doi.org/10.3389/fpls.2023.1090026
Pan et al. (2023). Stress-induced endocytosis from chloroplast inner envelope membrane is mediated by CHLOROPLAST VESICULATION but inhibited by GAPC.Cell Rep. 2023 Oct 31;42(10):113208. doi: 10.1016/j.celrep.2023.113208.
Fujimoto et al. (2020) Longin R-SNARE is retrieved from the plasma membrane by ANTH domain-containing proteins in Arabidopsis. Proc Natl Acad Sci U S A. 2020 Oct 6;117(40):25150-25158. doi: 10.1073/pnas.2011152117. Epub 2020 Sep 23. PMID: 32968023; PMCID: PMC7547277.
Ranjan et al. (2019). Transient Internalization and Microtubule-Dependent Trafficking of a Ciliary Signaling Receptor from the Plasma Membrane to the Cilium. Curr Biol. 2019 Aug 2. pii: S0960-9822(19)30867-X. doi: 10.1016/j.cub.2019.07.022.
Wattelet-Boyer et al. (2016). Enrichment of hydroxylated C24- and C26-acyl- chain sphingolipids mediates PIN2 apical sorting at trans-Golgi network subdomains. Nat Commun. 2016 Sep 29;7:12788. doi: 10.1038/ncomms12788.
Derbyshire et al. (2015). Proteomic Analysis of Microtubule Interacting Proteins over the Course of Xylem Tracheary Element Formation in Arabidopsis. Plant Cell. 2015 Oct 2. pii: tpc.15.00314.
Grones et al. (2015). Auxin-binding pocket of ABP1 is crucial for its gain-of-function cellular and developmental roles. J Exp Bot. 2015 Apr 28. pii: erv177.
McLoughlin et al. (2013). Identification of novel candidate phosphatidic acid binding proteins involved in the salt stress response of Arabidopsis thaliana roots. Biochem J. Jan 17. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Agrisera Educational Posters Collection - Reviews:
-
This product doesn't have any reviews.