1
Anti-PR-3 / CHN | Class I chitinase
AS07 207 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. stolonifera cv. ‘Penncross’, C. annuum, N. tabacum, P. abies, S. esculentum, S. lycopersicum, S. tuberosum, V. vinifera | Cellular marker of vacuolar contents
- Product Info
-
Immunogen: Purified tobacco class I chitinase. The preparation used is a mixture of two class I isoforms (Shinshi et al., 1990; van Buuren et al., 1992): 1) Chitinase A (CHN A) P08252 encoded by gene chn48 derived from the N. tomentosiformis ancestor of tobacco. 2) Chitinase B (CHN B) P24091 encoded by gene chn50 derived from the N. sylvestris ancestor of tobacco.
Host: Rabbit Clonality: Polyclonal Purity: Total IgG. Protein G purified in PBS pH 7.4. Format: Lyophilized Quantity: 2 mg Reconstitution: For reconstitution add 100 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Co-Immunoprecipitation (IP) (Co-IP), Immunolocalization (IL), Western blot (WB) Recommended dilution: 8 µg/ml (WB) Expected | apparent MW: 35, 34 | 32 and 34 kDa
- Reactivity
-
Confirmed reactivity: Agostis stolonifera cv. ‘Penncross’, Capsicum annuum, Nicotiana tabacum, Picea abies, Solanum esculentum, Solanum lycopersicum, Solanum tuberosum, Vitis vinifera
Predicted reactivity: Arabidopsis thaliana, Manihot esculenta, Zea mays
Species of your interest not listed? Contact usNot reactive in: No confirmed exceptions from predicted reactivity are currently known - Application Examples
-
Application example
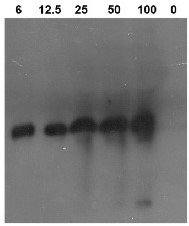
.jpg)
Detection of tobacco chitinase I in ng loaded per respective well using anti-tobacco chitinase I antibodies. Primary antibodies have been used at 8 µg/ml.
- Additional Information
-
Additional information: Antibody is recognizing closely related tobacco class I isoforms: endochitinase A CHN-A (ca. 34 kDa) and endochitinase B CHN-B (ca. 32 kDa)This antibody can be used as a marker of vacuolar contents Keefe et al. (1990). The effect of ethylene on the cell-type-specific and intracellular localization of β-1,3-glucanase and chitinase in tobacco leave. Plant 182: 43-51.Additional information (application): Important note: For blocking 5% skim milk in PBS without Ca++ should be used. This antibody is purified by affinity chromarography on Portein G. - Background
-
Background: Pathogenesis-related (PR) proteins, are induced in response to the infection of plants with microbial pathogens. Combinations of glucanase I and chitinase I are potent inhibitors of fungal growth in vitro however precise mechanism of that is still not known. Glucanase I (PR-2) and chitinase I (PR-3) contribute to defense against fungal infection and are currently used as markers for innate immunity, and in particular the ethylene/jasmonate signalling pathway in pathogenesis.
- Product Citations
-
Selected references: Mansilla et al. (2020).- Characterization of functionalized bentonite as nanocarrier of salicylic acid with protective action against Pseudomonas syringae in tomato plants. Eur J Plant Pathol 158, 211?222 (2020). https://doi.org/10.1007/s10658-020-02067-w
Colman et al. (2019). Chitosan microparticles improve tomato seedling biomass and modulate hormonal, redox and defense pathways. Plant Physiology and Biochemistry Volume 143, October 2019, Pages 203-211.
Kumari et al. (2017), Overexpression of a Plasma Membrane Bound Na+/H+ Antiporter-Like Protein (SbNHXLP) Confers Salt Tolerance and Improves Fruit Yield in Tomato by Maintaining Ion Homeostasis. Front Plant Sci. 2017 Jan 6;7:2027. doi: 10.3389/fpls.2016.02027.
Jespersen et al. (2017). Metabolic Effects of Acibenzolar-S-Methyl for Improving Heat or Drought Stress in Creeping Bentgrass. Front Plant Sci. 2017 Jul 11;8:1224. doi: 10.3389/fpls.2017.01224. eCollection 2017. (western blot, Agostis stolonifera cv. ?Penncross?)
Ko et al. (2016). Constitutive expression of a fungus-inducible carboxylesterase improves disease resistance in transgenic pepper plants. Planta. 2016 Aug; 244(2):379-92. doi: 10.1007/s00425-016-2514-6. Epub 2016 Apr 13.
Anil Kumar et al. (2016). Beyond just being foot soldiers – osmotin like protein (OLP) and chitinase (Chi11) genes act as sentinels to confront salt, drought, and fungal stress tolerance in tomato. Environmental and Experimental Botany 132 (2016) 53–65.
Wu et al. (2016). Laminarin modulates the chloroplast antioxidant system to enhance abiotic stress tolerance partially through the regulation of the defensin-like gene expression. Plant Science, Volume 247, June 2016, Pages 83–92.
Falcioni et al. (2013). Effect of salicylic acid treatment on tomato plant physiology and tolerance to potato virus X infection. Eur J Plant Pathol DOI 10.1007/s10658-013-0333-1.
Munger et al. (2012). Beneficial 'unintended effects' of cereal cystatin in transgenic lines of potato, Solanum tuberosum. BMC Plant Biology, 12:198.
Sticher et al. (1993). Posttranslational processing of a new class of hydroxyproline-containing proteins: Prolyl hydroxylation and C-terminal cleavage of tobacco (Nicotiana tabacum) vacuolar chitinase. Plant Physiol. 101, 1239-1247. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts - Reviews:
-
This product doesn't have any reviews.
Accessories
AS07 208 | Clonality: Polyclonal | Host: Rabbit | Reactivity: N. benthamiana, N. clevilandii, N. tabacum, Phalenopsis, P.abies, S.lycopersicum, S. tuberosum, V. vinifera | compartment marker of vacuolar contents


