1

Anti-PR-1 | Pathogenesis-related protein 1
AS10 687 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. thaliana, H. vulgare, N. bentamiana, S. oleracea, S. lycopersicum, T. aestivum, Z. mays, V. vinifera
- Product Info
-
Immunogen: N-terminal part of recombinant PR-1 protein from Arabidopsis thaliana UniProt: P33154, TAIR: At2g14610
Host: Rabbit Clonality: Polyclonal Purity: Serum Format: Lyophilized Quantity: 50 µl Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Western blot (WB) Recommended dilution: 1 : 2500 (WB) Expected | apparent MW: 17.7 kDa (Arabidopsis thaliana)
- Reactivity
-
Confirmed reactivity: Arabidopsis thaliana, Hordeum vulgare, Nicotiana bentamiana, Spinacia oleracea, Solanum lycopersicum, Triticum aestivum, Vitis vinifera, Zea mays Predicted reactivity: Actinidia deliciosa, Capsicum annuum, Glycine max
Species of your interest not listed? Contact usNot reactive in: Citrus sinensis, - Application Examples
-
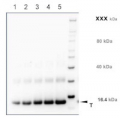
Application example

Recombinant PR-1 protein standard 0.05 pmol (1), 0.1 pmol (2), 0.15 pmol (3), 0.2 pmol (4), and 0.3 pmol (5) was loaded in each lane. Protein separation was done using NuPage 4-12% Tris-Bis gel (Invitrogen) LDS-PAGE and blotted 1h to PVDF. Blots were blocked immediately following transfer in 2-2.5 % RPN2125 (GE Healthcare) in 20 mM Tris, 137 mM sodium chloride pH 7.6 with 0.1% (v/v) Tween-20 (TBS-T) for 1h at room temperature with agitation. Blots were incubated in the primary anti-PR-1 antibody at a dilution of 1: 10 000 in blocking reagent for 1h at room temperature with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at room temperature with agitation. Blots were incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, recommended secondary antibody AS09 602) diluted to 1:25 000 in TBS-T for 1h at room temperature with agitation. The blots were washed as above and developed for 5 min with chemiluminescent detection reagent in extreme low femtogram range, according the manufacturers instructions. Images of the blots were obtained using a CCD imager (FluorSMax, Bio-Rad) and Quantity One software (Bio-Rad). Exposure time was 1 minute.
Immunolocalization
Immunostaining of Arabidopsis thaliana seedlings treated with 250 µM SA for 120 minutes for induction of PR- gene expression (right panel) or control without SA (left panel). Steps involved: fixation: in 2 % formaldehyde in MTSB buffer for 30 minutes at 37 °C; washing with water, hydrophilization with methanol; cell wall digestion: 5-7 minutes, 0.25% Dricelaze, 0.1 % Macerozyme in 5 mM MES buffer, pH5.2; cell wall permeabilization: 10 % DMSO/3 % NP40 in MTSB buffer; primary antibody incubation: dilution 1: 200 in MTBS buffer for 3 h at RT; secondary antibody incubation: dilution 1: 1000 at RT for 1 h, goat-anti rabbit IgG Alexa 488 conjugated antibody.
Courtesy of Dr. Taras Pasternak, Freiburg University, Germany - Additional Information
-
Additional information: PR-1 protein is present in very low amonts in non-induced plant material.
Overnight antibody incubation is not recommended.
This product can be sold containing Proclin if requested.Additional information (application): Re-using of antibody solution is not recommended, It will contribute to incrteased background signal - Background
-
Background: Pathogenesis-related protein 1 (PR-1), small antimicrobial protein which acts as a marker of plant immune signaling and is partially responsible for acquired pathogen resistance. Induced by INA, salicylic acid and pathogen infection.
- Product Citations
-
Selected references: Cook et al. (2024). Chloroplast phosphatases LPPγ and LPPε1 facilitate conversion of extraplastidic phospholipids to galactolipids. Plant Physiol. 2024 May 31;195(2):1506-1520. doi: 10.1093/plphys/kiae100.
Garcia-Murillo et al. (2023) CRISPRa-mediated transcriptional activation of the SlPR-1 gene in edited tomato plants, Plant Science,Volume 329,2023, 111617, ISSN 0168-9452,https://doi.org/10.1016/j.plantsci.2023.111617.
Pecenkova et al. (2022) Immunity functions of Arabidopsis pathogenesis-related 1 are coupled but not confined to its C-terminus processing and trafficking. Mol Plant Pathol. 2022 May;23(5):664-678. doi: 10.1111/mpp.13187. Epub 2022 Feb 4. PMID: 35122385; PMCID: PMC8995067.
Baena et al. (2022) SNARE SYP132 mediates divergent traffic of plasma membrane H+-ATPase AHA1 and antimicrobial PR1 during bacterial pathogenesis. Plant Physiol. 2022 Jun 27;189(3):1639-1661. doi: 10.1093/plphys/kiac149. PMID: 35348763; PMCID: PMC9237740.
Bhandari et al. (2023). Defense against phytopathogens relies on efficient antimicrobial protein secretion mediated by the microtubule-binding protein TGNap1. at Commun. 2023 Oct 11;14(1):6357. doi: 10.1038/s41467-023-41807-4.
Wang et al. (2023). Plant mRNAs move into a fungal pathogen via extracellular vesicles to reduce infection. Cell Host Microbe. 2023 Dec 13:S1931-3128(23)00470-5.doi: 10.1016/j.chom.2023.11.020.
Chen et al. (2023). XCP1 cleaves Pathogenesis-related protein 1 into CAPE9 for systemic immunity in Arabidopsis. Nat Commun. 2023 Aug 4;14(1):4697.doi: 10.1038/s41467-023-40406-7.
Li et al. (2020). N-terminal acetylation stabilizes SIGMA FACTOR BINDING PROTEIN 1 involved in salicylic acid-primed cell death. Plant Physiol. 2020 Mar 5. pii: pp.01417.2019. doi: 10.1104/pp.19.01417.
Jung et al. (2020). Pathogen-associated Molecular Pattern-triggered Immunity Involves Proteolytic Degradation of Core Nonsense-mediated mRNA Decay Factors During the Early Defense Response. Plant Cell, February 2020.
Chang et al. (2019). PBS3 Protects EDS1 from Proteasome-Mediated Degradation in Plant Immunity. Mol Plant. 2019 Feb 11. pii: S1674-2052(19)30055-3. doi: 10.1016/j.molp.2019.01.023.
Lv et al. (2019). Uncoupled Expression of Nuclear and Plastid Photosynthesis-Associated Genes Contributes to Cell Death in a Lesion Mimic Mutant. Plant Cell. 2019 Jan;31(1):210-230. doi: 10.1105/tpc.18.00813.
Cecchini et al. (2018). Underground azelaic acid-conferred resistance to Pseudomonas syringae in Arabidopsis. Mol Plant Microbe Interact. 2018 Aug 29. doi: 10.1094/MPMI-07-18-0185-R.
Chakraborty et al. (2018). Epigenetic and transcriptional control of chickpea WRKY40 promoter activity under Fusarium stress and its heterologous expression in Arabidopsis leads to enhanced resistance against bacterial pathogen. Plant Science, doi.org/10.1016/j.plantsci.2018.07.014
Izquierdo et al. (2018). Arabidopsis nonresponding to oxylipins locus NOXY7 encodes a yeast GCN1 homolog that mediates noncanonical translation regulation and stress adaptation. Plant Cell Environ. 2018 Mar 2. doi: 10.1111/pce.13182.
Seguel et al. (2018). PROHIBITIN 3 forms complexes with ISOCHORISMATE SYNTHASE 1 to regulate stress-induced salicylic acid biosynthesis in Arabidopsis. Plant Physiol. Jan 2018. DOI:10.1104/pp.17.00941
Huh et al. (2017). Protein-protein interactions in the RPS4/RRS1 immune receptor complex. PLoS Pathog. 2017 May 5;13(5):e1006376. doi: 10.1371/journal.ppat.1006376.
Zhang et al. (2017). A suite of receptor-like kinases and a putative mechano-sensitive channel are involved in autoimmunity and plasma membrane-based defenses in Arabidopsis. Mol Plant Microbe Interact. 2017 Jan 4. doi: 10.1094/MPMI-09-16-0184-R.
Zhu et al. (2016). CML8, an Arabidopsis calmodulin-like protein plays a role in Pseudomonas syringae plant immunity. Plant Cell Physiol. 2016 Nov 10. pii: pcw189. [Epub ahead of print] - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Agrisera Educational Posters Collection
- Reviews:
-
Joanna Jelenska | 2021-04-14This antibody works very well, very strong signal and no background. Our lab used this antibody in many experiments to show defense induction by pathogens and PAMPs in Arabidopsis, e.g. https://academic.oup.com/plphys/article/176/3/2515/6117135
Ben Field | 2019-02-25We have excellent results with this antibody- a strong signal with low background on Arabidopsis total protein extracts. Recommended.