1
Anti-BiP | Lumenal-binding protein (rabbit antibody)
AS09 481 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. thaliana, B. napus, Chara australis, C. reinhardtii, C. sativus, M. perniciosa, N. benthamiana, N. tabacum, R. sativa, L.Tokinashi-daikon, O. europaea, O. sativa, P. abies, P. patens, S. oleracea, S. lycopersicum, S. tuberosum, T. aestivum, Z. mays
- Product Info
-
Immunogen: KLH-conjugated synthetic peptide derived from Arabidopsis thaliana BiP proteins: BiP1 At5g28540 Q9LKR3, BiP2 At5g42020 F4K007 , BiP3 At1g09080 Q8H1B3
Host: Rabbit Clonality: Polyclonal Purity: Immunogen affinity purified serum in PBS pH 7.4. Format: Lyophilized Quantity: 50 µg Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: ELISA (ELISA), Immunofluorescence (IF), Immunogold (IG), Western blot (WB) Recommended dilution: 1 : 8000 (ELISA), 1 : 600 (IF), 1 : 2000 (WB) Expected | apparent MW: 73.5 | 80 kDa - Reactivity
-
Confirmed reactivity: Anacardium occidentale, Arabidopsis thaliana, Brassica napus,Chara australis R.Br, Chlamydomonas reinhardtii, Cucumis sativus, Mangifera indica, Moniliophthora perniciosa, Nicotiana benthamiana, Nicotiana tabacum, Raphanus sativa L. Tokinashi-daikon, Olea europaea, Oryza sativa, Picea abies, Pistachio sp., Physcomitrium patens, Schinus molle, Spinacia oleracea, Solanum lycopersicum, Solanum tuberosum, Triticum aestivum, Zea mays Predicted reactivity: Arabis alpina, Capsella rubella, Capsicum annuum, Citrus clementina, Citrus sinsensis, Eucalyptus grandis, Glycine max, Hordeum vulgare, Isatis tincorina, Prunus persica, Triticum aestivium, Petunia hybrida, Picea sitcHensis, Populus trichocarpa, Ricinus comminus, Vitis vinifera
Species of your interest not listed? Contact usNot reactive in: Ostreococcus tauri, Schizosaccharomyces pombe - Application Examples
-
.jpg)
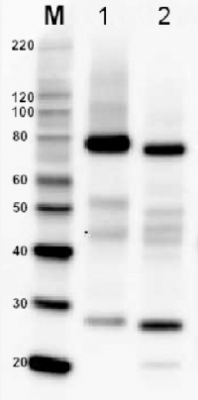
5 µg of total protein from A.thaliana (1), H. vulgare (2), P. sativum (3)*, Z. mays (4), C. sativus(5), S. tuberosum (6), S. oleracea (7), S. lycopersicum (8) P. patens (9)*, C. reinhardtii (10) extracted with Agrisera PEB extraction buffer (AS08 300) were separated on 4-12% SDS-PAGE and blotted 1h to PVDF. Blots were blocked immediately following transfer in 5 % non-fat milk in TBS-T, for 1h at room temperature with agitation. Blots were incubated in the primary antibody at a dilution of 1: 10 000 for 1h at room temperature with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at room temperature with agitation. Blots were incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, from Agrisera AS09 602) diluted to 1:50 000 for 1h at room temperature with agitation. The blots were washed as above and developed for 5 min with ECL detection reagent of extreme femtogram range, according to the manufacturers instructions. Exposure time was 5 seconds. * Lack of the signal or its low signal intensity in those samples can be due to the sample biology. If you work with those species, please inquire.
Immunolocalization

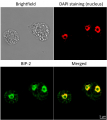
BiP localization in 5 days old Arabidopsis thaliana roots (A), 3 days old Triticum aestivum roots (B).
BiP signal shown in red, DAPI in blue. The material has been fixed in para-formaldehyde for 30 minutes. Tissue cleaning has been performed before immunolocalization. Rabbit anti-BiP primary antibody diluted in 1: 600 and ALEXA 555 conjugated anti-rabbit secondary antibody (red color) have been used. Co-staining with DAPI visualized nucleus (blue color). Scale bar – 10 µm.Courtesy Dr. Taras Pasternak, Freiburg University, Germany
Application examples: 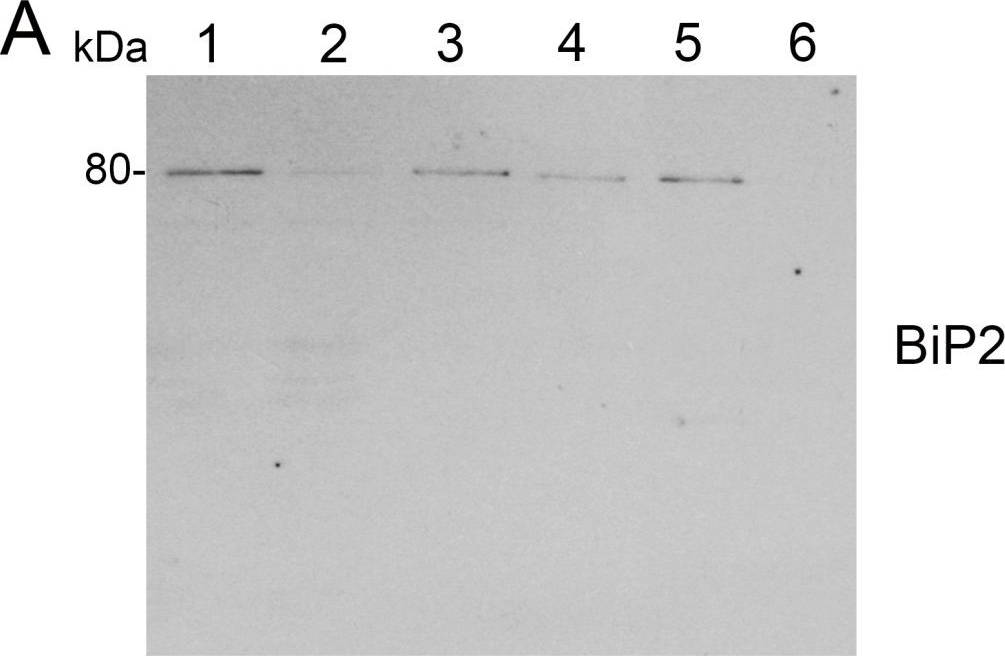
Reactant: Malus domestica (Apple)
Application: Western Blotting
Pudmed ID: 23886449
Journal: Plant Methods
Figure Number: 2A
Published Date: 2013-07-28
First Author: Sikorskaite, S., Rajamäki, M. L., et al.
Impact Factor: 5.139
Open PublicationAnalysis of protein fractions from different steps of nuclei isolation. The protein fractions obtained following the main steps of the procedure used for isolation of nuclei from leaves of apple are shown. Equal amounts of proteins (4 ?g) from each fraction were loaded on the gel. A) Lumenal-binding protein 2 (BiP2), B) histone H3 and C) plastocyanin (PC) were detected with specific antibodies by western blot analysis. D) Coomassie blue staining of the fractionated proteins separated by polyacrylamide gel electrophoresis. Lane 1: crude extract of proteins from homogenized leaf tissue; lane 2: resuspended pellet of the whole cell lysate obtained following treatment with Triton X-100 and centrifugation; lane 3: nuclei collected from the 60% Percoll layer (see nuclear fraction in Figure 1B); lane 4: the interface fraction of 60% Percoll and 2.5 M sucrose layers in the density gradient containing chloroplasts and unbroken cells (see 60% P/2.5 M S interface in Figure 1B); lane 5: sucrose layer of the density gradient (see 2.5 M sucrose layer in Figure 1B); lane 6: nuclei purified by centrifugation on a 35% Percoll cushion.
Reactant: Solanum lycopersicum (Tomato)
Application: Western Blotting
Pudmed ID: 26347766
Journal: Front Plant Sci
Figure Number: 6A
Published Date: 2015-09-09
First Author: Buxa, S. V., Degola, F., et al.
Impact Factor: 5.435
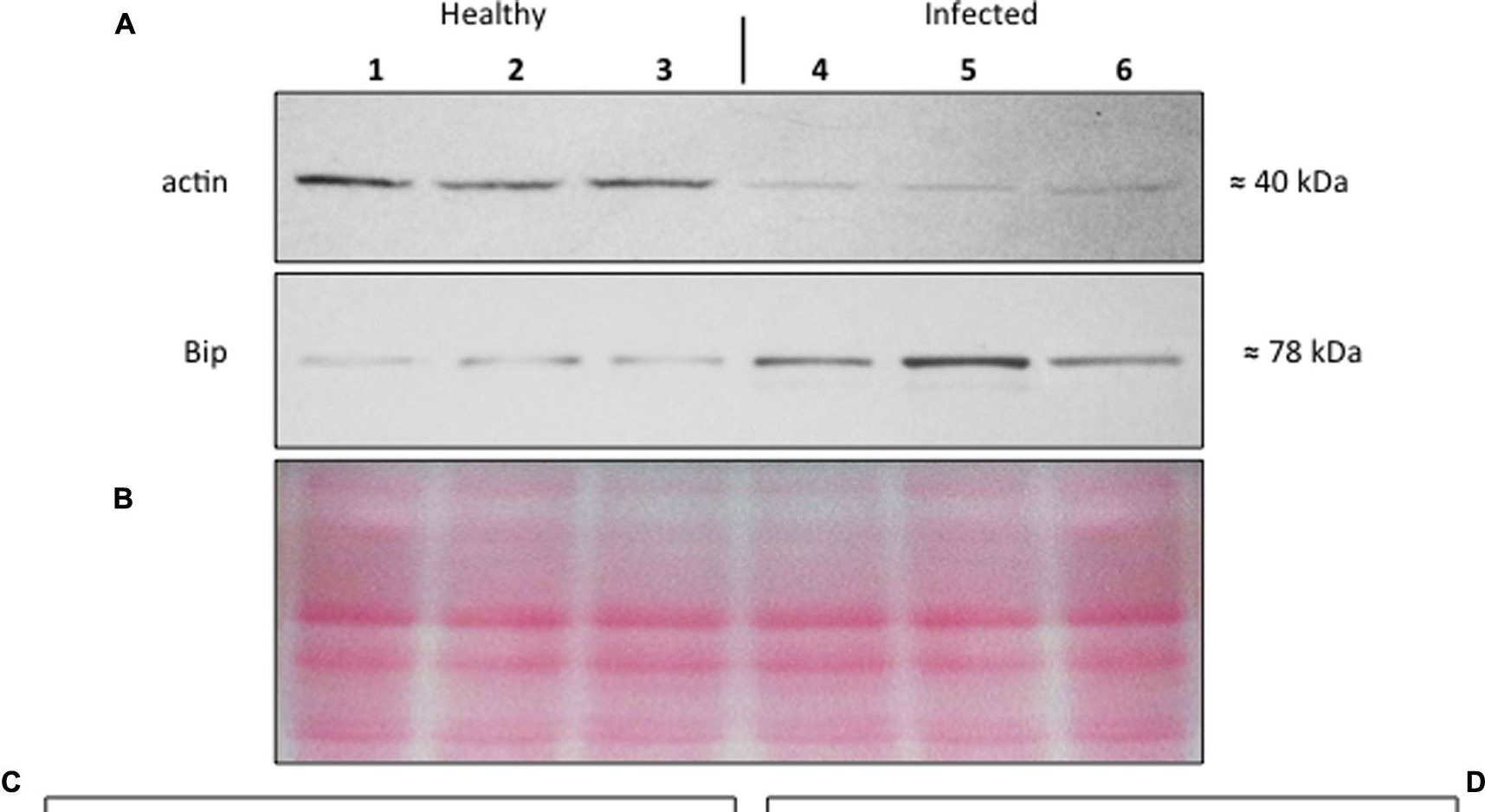
Open Publication(A–D) Western blot analyses of S. lycopersicum midrib extracts. Three healthy (lanes 1–3) and three infected (lanes 4–6) plants were analyzed. Immunoreactive signals corresponding to actin and ER luminal binding protein (BiP) displayed the expected molecular weights of ?40 and ?78 kDa, respectively (A). Amount of loaded protein were checked by Ponceau-S staining (B). Quantification of immunoreactive luminescence signals was achieved by densitometric scanning of the respective actin (C) and BiP (D) bands. The mean of six replicates (±SD) was calculated for each sample. Actin T-value: –9.977; BiP T-value: 15.068. * denotes significant difference of the means (P-values ? 0.001).
Reactant: Moniliophthora perniciosa (Witches' broom disease)
Application: Western Blotting
Pudmed ID: 28818052
Journal: BMC Microbiol
Figure Number: 4A
Published Date: 2017-08-17
First Author: Mares, J. H., Gramacho, K. P., et al.
Impact Factor: 3.437
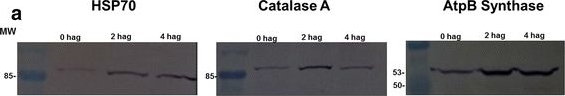
Open PublicationAccumulation of BiP, Catalase, and ATP synthase (beta chain) by western blot and analysis of spots. a Image of a nitrocellulose membrane hybridized with Anti-BiP, Anti-Catalase, and Anti-ATP synthase (beta chain) of Arabidopsis (Normalization, see Additional file 6: Figure S4). b Relative accumulation determined from images of the nitrocellulose membrane, using the Gel Quant. Net 1.8.0 software. c Relative quantification of each spot corresponding to proteins analyzed by western blot. The average values of the each spot’s percentage volume in each gel replicate was used for graph construction
Reactant: Chlamydomonas reinhardtii (Green Alga)
Application: Western Blotting
Pudmed ID: 32731621
Journal: Cells
Figure Number: 6A
Published Date: 2020-07-28
First Author: Garrido, C., Caspari, O. D., et al.
Impact Factor: None
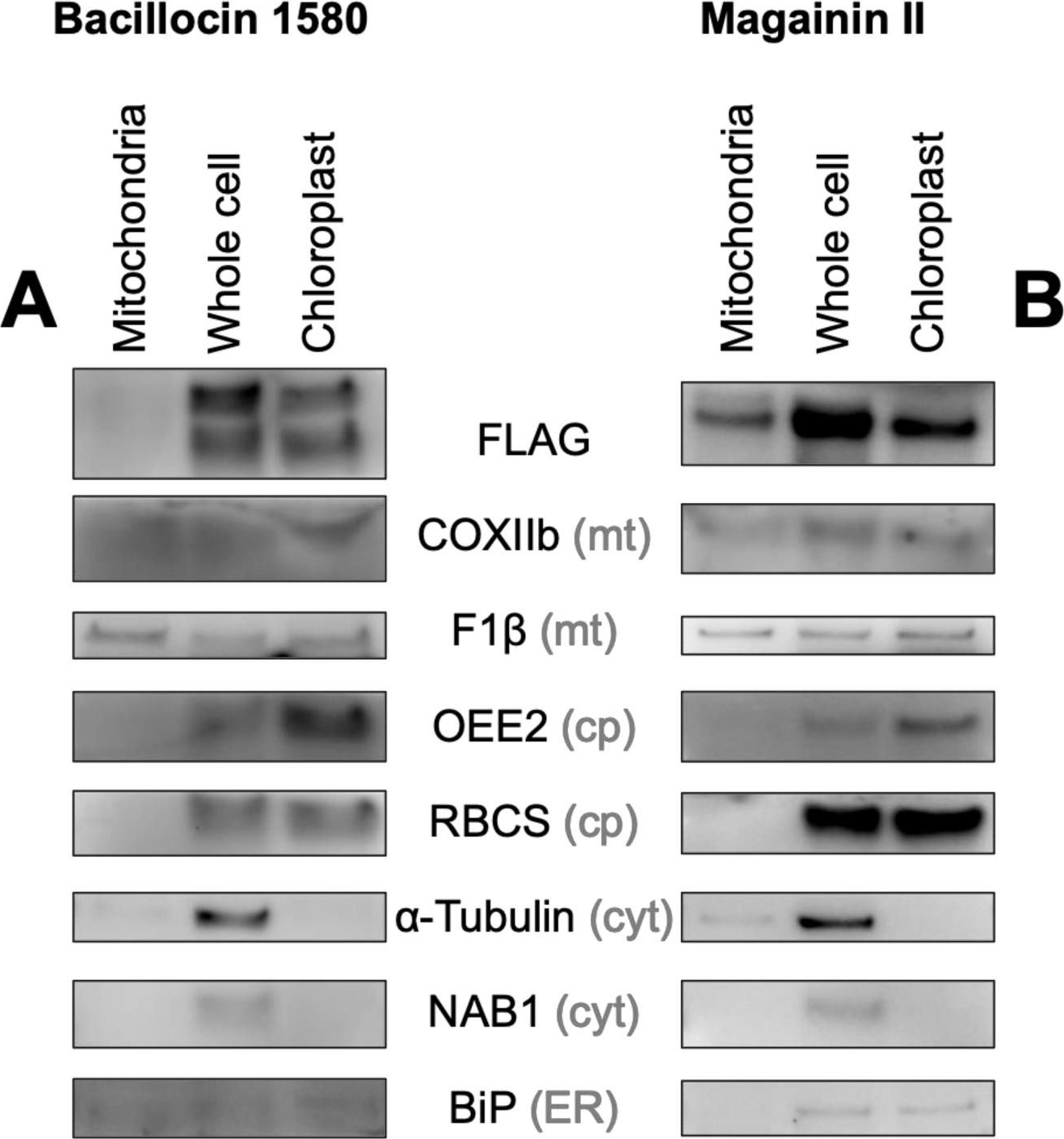
Open PublicationBiochemical confirmation of AMP targeting activity. Mitochondrial, whole cell and chloroplast fractions (1 ?g protein per well) isolated from Chlamydomonas strains in which Venus localization is driven by bacillocin 1580 (A) or magainin II (B), each fused to the RBCA cleavage site, were immunolabelled with antibodies raised against FLAG, an epitope tag carried C-terminally by the Venus reporter, and markers for different cellular compartments: Cytochrome Oxidase subunit IIb (COXIIb) and ATPsynthase subunit F1? for mitochondria (mt), Photosystem II Oxygen Evolving Enhancer 2 (OEE2) and Rubisco small subunit (RBCS) for chloroplasts (cp), ?-Tubulin and nucleic-acid binding protein 1 (NAB1) for the cytosol (cyt) and luminal binding protein (BiP) for the endoplasmic reticulum (ER). Isolated chloroplasts from the Bacillocin 1580 strain (C) and isolated mitochondria from the Magainin II strain (D) were subjected to a proteinase assay, where aliquots were treated with either 150 ?g mL?1 proteinase K and/or 1% Triton X-100, a membrane solubilizing detergent. Aliquots were subsequently immuno-labelled with antibodies against FLAG, chloroplast ATP synthase subunit CF1 ? and other organelle-markers described aside.
Reactant: Chlamydomonas reinhardtii (Green Alga)
Application: Western Blotting
Pudmed ID: 32731621
Journal: Cells
Figure Number: 6C
Published Date: 2020-07-28
First Author: Garrido, C., Caspari, O. D., et al.
Impact Factor: None
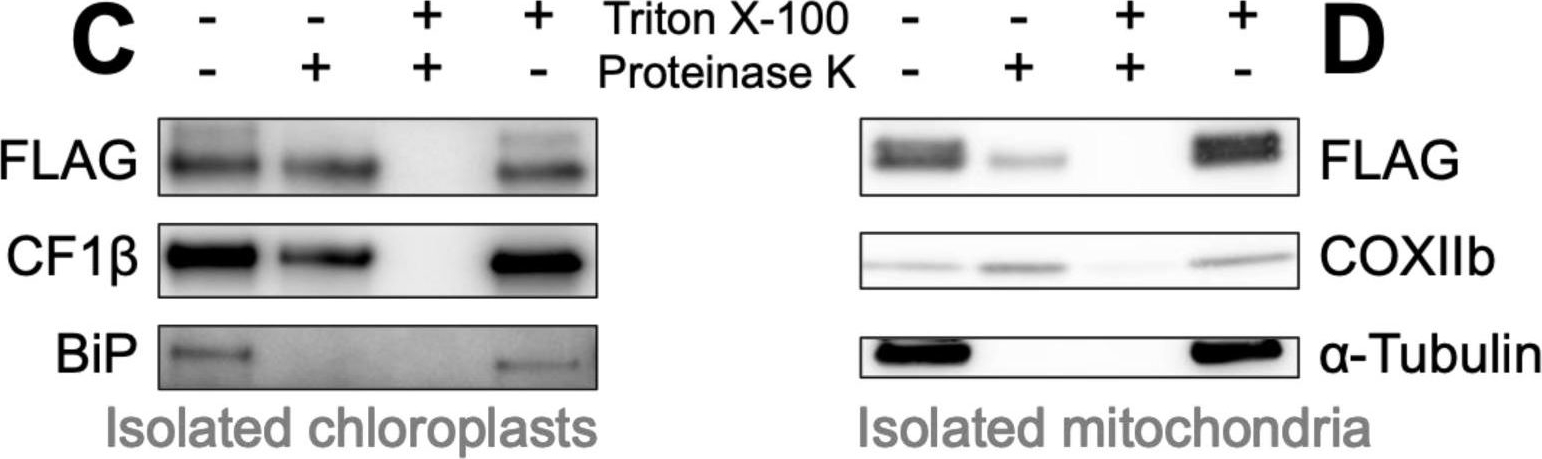
Open PublicationBiochemical confirmation of AMP targeting activity. Mitochondrial, whole cell and chloroplast fractions (1 ?g protein per well) isolated from Chlamydomonas strains in which Venus localization is driven by bacillocin 1580 (A) or magainin II (B), each fused to the RBCA cleavage site, were immunolabelled with antibodies raised against FLAG, an epitope tag carried C-terminally by the Venus reporter, and markers for different cellular compartments: Cytochrome Oxidase subunit IIb (COXIIb) and ATPsynthase subunit F1? for mitochondria (mt), Photosystem II Oxygen Evolving Enhancer 2 (OEE2) and Rubisco small subunit (RBCS) for chloroplasts (cp), ?-Tubulin and nucleic-acid binding protein 1 (NAB1) for the cytosol (cyt) and luminal binding protein (BiP) for the endoplasmic reticulum (ER). Isolated chloroplasts from the Bacillocin 1580 strain (C) and isolated mitochondria from the Magainin II strain (D) were subjected to a proteinase assay, where aliquots were treated with either 150 ?g mL?1 proteinase K and/or 1% Triton X-100, a membrane solubilizing detergent. Aliquots were subsequently immuno-labelled with antibodies against FLAG, chloroplast ATP synthase subunit CF1 ? and other organelle-markers described aside.
- Additional Information
-
Additional information (application): Protein or membrane sample should be treated at 70°C for 10 min before loading on the gel, This antibody has so far not worked in IP - Background
-
Background: BiP2 (Binding immunoglobulin protein) is localized in endoplasmic reticulum lumen (ER) and plays a role in protein assembly inside ER. BiP protein is abundant under all growth conditions but its synthesis can increase under conditions that lead to the accumulation of unfolded polypeptides in endoplasmic reticulum (ER).
- Product Citations
-
Selected references: Zhou et al. (2024). Antagonistic systemin receptors integrate the activation and attenuation of systemic wound signaling in tomato. Dev Cell. 2024 Nov 26:S1534-5807(24)00670-1. doi: 10.1016/j.devcel.2024.11.005.
Salomon et al. (2024). Betaine lipids overproduced in seed plants are excluded from plastid membranes and promote endomembrane expansion. J Exp Bot. 2024 Nov 14:erae458. doi: 10.1093/jxb/erae458.
Li et al. (2024). Membrane protein MHZ3 regulates the on-off switch of ethylene signaling in rice. Nat Commun. 2024 Jul 16;15(1):5987. doi: 10.1038/s41467-024-50290-4.
Miloro et al. (2024). Barley AGO4 proteins show overlapping functionality with distinct small RNA-binding properties in heterologous complementation. Plant Cell Rep. 2024 Mar 13;43(4):96. doi: 10.1007/s00299-024-03177-z.
Xue et al.(2023). The PtdIns3P phosphatase MtMP promotes symbiotic nitrogen fixation via mitophagy in Medicago truncatula. iScience. 2023 Sep 15;26(10):107752.doi: 10.1016/j.isci.2023.107752.
Guo, Zhang , Wang, et al. (2023) Cold-induced calreticulin OsCRT3 conformational changes promote OsCIPK7 binding and temperature sensing in rice. EMBO J. 2023;42(1):e110518. doi:10.15252/embj.2021110519
Jiang et al. (2022) CEF3 is involved in membrane trafficking and essential for secondary cell wall biosynthesis and its mutation enhanced biomass enzymatic saccharification in rice. Biotechnol Biofuels Bioprod. 2022;15(1):111. Published 2022 Oct 14. doi:10.1186/s13068-022-02205-y
Baena et al. (2022) SNARE SYP132 mediates divergent traffic of plasma membrane H+-ATPase AHA1 and antimicrobial PR1 during bacterial pathogenesis. Plant Physiol. 2022 Jun 27;189(3):1639-1661. doi: 10.1093/plphys/kiac149. PMID: 35348763; PMCID: PMC9237740.
Hacquard et al. (2022) The Arabidopsis F-box protein FBW2 targets AGO1 for degradation to prevent spurious loading of illegitimate small RNA. Cell Rep. 2022 Apr 12;39(2):110671. doi: 10.1016/j.celrep.2022.110671. PMID: 35417704; PMCID: PMC9035678.
Skalicky et al. (2021) Auxin Metabolite Profiling in Isolated and Intact Plant Nuclei. Int J Mol Sci. 2021 Nov 16;22(22):12369. doi: 10.3390/ijms222212369. PMID: 34830250; PMCID: PMC8620152.
Gu et al. (2021) A Lipid Bodies-Associated Galactosyl Hydrolase Is Involved in Triacylglycerol Biosynthesis and Galactolipid Turnover in the Unicellular Green Alga Chlamydomonas reinhardtii
Dittmer, Kleine, & Schwenkert. (2021) The TPR- and J-domain-containing proteins DJC31 and DJC62 are involved in abiotic stress responses in Arabidopsis thaliana. J Cell Sci. 2021 Oct 1;134(19):jcs259032. doi: 10.1242/jcs.259032. Epub 2021 Oct 12. PMID: 34515300.
Shteinberg et al. (2021) Tomato Yellow Leaf Curl Virus (TYLCV) Promotes Plant Tolerance to Drought. Cells. 2021 Oct 25;10(11):2875. doi: 10.3390/cells10112875. PMID: 34831098; PMCID: PMC8616339.
Mishra et al. (2021) Interplay between abiotic (drought) and biotic (virus) stresses in tomato plants. Mol Plant Pathol. 2021 Dec 30. doi: 10.1111/mpp.13172. Epub ahead of print. PMID: 34970822.
Yang et al. (2020). PROTEIN PHOSPHATASE 95 Regulates Phosphate Homeostasis by Affecting Phosphate Transporter Trafficking in Rice. Plant Cell. 2020 Jan 9. pii: tpc.00685.2019. doi: 10.1105/tpc.19.00685.
Hurny et al. (2020). SYNERGISTIC ON AUXIN AND CYTOKININ 1 Positively Regulates Growth and Attenuates Soil Pathogen Resistance. Nat Commun. 2020 May 1;11(1):2170. doi: 10.1038/s41467-020-15895-5. (immunolocalization)
Jang et al. (2020). 1Molecules and CellsCrABCA2 Facilitates Triacylglycerol Accumulation in Chlamydomonas reinhardtii under Nitrogen Starvation. Mol Cells. 2020 Jan 31;43(1):48-57. doi: 10.14348/molcells.2019.0262.
Zhang et al. (2019) Arabinosyl Deacetylase Modulates the Arabinoxylan Acetylation Profile and Secondary Wall Formation. Plant Cell. 2019 May;31(5):1113-1126. doi: 10.1105/tpc.18.00894. Epub 2019 Mar 18. PMID: 30886126; PMCID: PMC6533017.
Mares et al. (2020). Hydrosoluble phylloplane components of Theobroma cacao modulate the metabolism of Moniliophthora perniciosa spores during germination.Fungal Biol. 2020 Jan;124(1):73-81. doi: 10.1016/j.funbio.2019.11.008.
Dalmadi et al. (2019). AGO-unbound cytosolic pool of mature miRNAs in plant cells reveals a novel regulatory step at AGO1 loading. Nucleic Acids Res. 2019 Aug 8. pii: gkz690. doi: 10.1093/nar/gkz690.
Feng et al. (2019). Analyses of transgenic fibroblast growth factor 21 mature rice seeds. J-STAGe, Online ISSN : 1347-3735.
Bastiaan-Net et al. (2018). IgE Cross-Reactivity of Cashew Nut Allergens. Int Arch Allergy Immunol. 2018 Oct 26:1-14. doi: 10.1159/000493100.
Wang et al. (2018). Resistance protein Pit interacts with the GEF OsSPK1 to activate OsRac1 and trigger rice immunity. Proc Natl Acad Sci U S A. 2018 Nov 16. pii: 201813058. doi: 10.1073/pnas.1813058115.
Pertl-Obermeyer et al. (2018). Dissecting the subcellular membrane proteome reveals enrichment of H+ (co-)transporters and vesicle trafficking proteins in acidic zones of Chara internodal cells. PLoS One. 2018 Aug 29;13(8):e0201480. doi: 10.1371/journal.pone.0201480.
Qiao et al. (2018). Two Crinivirus-Conserved Small Proteins, P5 and P9, Are Indispensable for Efficient Lettuce infectious yellows virus Infectivity in Plants. Viruses. 2018 Aug 28;10(9). pii: E459. doi: 10.3390/v10090459.
Mares et al. (2017). Proteomic analysis during of spore germination of Moniliophthora perniciosa, the causal agent of witches' broom disease in cacao. BMC Microbiol. 2017 Aug 17;17(1):176. doi: 10.1186/s12866-017-1085-4.
Gelova et al. (2017). Antibody-mediated modulation of cytokinins in tobacco: organ-specific changes in cytokinin homeostasis. J Exp Bot. 2017 Dec 23. doi: 10.1093/jxb/erx426.
Nagel et al. (2017). Arabidopsis SH3P2 is an ubiquitin-binding protein that functions together with ESCRT-I and the deubiquitylating enzyme AMSH3. Proc Natl Acad Sci U S A. 2017 Aug 7. pii: 201710866. doi: 10.1073/pnas.1710866114.
Lomin et al. (2017). Studies of cytokinin receptor–phosphotransmitter interaction provide evidences for the initiation of cytokinin signalling in the endoplasmic reticulum. Functional Plant Biology, CSIRO Publications. (Nicotiana benthamiana, western blot)
Zhang et al. (2017). Control of secondary cell wall patterning involves xylan deacetylation by a GDSL esterase. Nat Plants. 2017 Mar 3;3:17017. doi: 10.1038/nplants.2017.17. (Oryza sativa, immunolocalization, western blot)
Je et al. (2016). Signaling from maize organ primordia via FASCIATED EAR3 regulates stem cell proliferation and yield traits. Nat Genet. 2016 Jul;48(7):785-91. doi: 10.1038/ng.3567. Epub 2016 May 16. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Agrisera Educational Posters CollectionMethod of isolation of plant ER


Courtesy of Dr. Masayoshi Maeshima, Laboratory of Cell Dynamics, Graduate School of Bioagricultural Sciences Nagoya University Nagoya, Japan
- Reviews:
-
Alberto Dávalos | 2024-04-12This antibody worked very well for A. thaliana Extracellular Vesicles and from Brassica oleracea (broccoli) EVs. Dilution 1:2000 in 10% SDS PAGEPeter Ma | 2021-02-04This antibody works very well for our samples. The membrane proteins from the Arabidopsis roots are subjected to 10% SDS-PAGE and then detected with this antibody (1:2000). The results shows that this antibody is quite specific and sensitive.Francesca | 2014-11-09We successfully used Bip antibody diluted 1:10000 against S. lycopersicon leaves extracts. Probing was conducted for 1h in PBS 5% skim milk.Sidona | 2011-11-16We have used BiP2 antibody with protein extract from Nicotiana and Malus species at dilution 1:2000 . The same dilution could be re-used 2 times.